| Synonyms |
Trandolapril (JAN/INN); Trandolapril [INN:BAN]; Trandolaprilum; Trandolaprilum [Latin]; Gopten; Mavik (TN); Preran; RU 44570; RU-44570; RU44570; (2S,3aR,7aS)-1-((S)-N-((S)-1-Carboxy-3-phenylpropyl)alanyl)hexahydro-2-indolinecarboxylic acid, 1-ethyl ester; (2S,3aR,7aS)-1-(N-((1S)-1-((Ethyloxy)carbonyl)-3-phenylpropyl)-L-alanyl)octahydro-1H-indole-2-carboxylic Acid; (2S,3aR,7aS)-1-[(2S)-2-[[(2S)-1-ethoxy-1-oxo-4-phenylbutan-2-yl]amino]propanoyl]-2,3,3a,4,5,6,7,7a-octahydroindole-2-carboxylicacid; 1-(2-((1-(ethoxycarbonyl)-3-phenylpropyl)amino)-1-oxopropyl)octahydro-1H-indol-2-carboxylic acid; Mavik; Odric; Odrik; Udrik
|
| Cross-matching ID |
- PubChem CID
- 5484727
- PubChem SID
-
7847449
; 7980822
; 12013675
; 14807551
; 14929957
; 17185447
; 39472377
; 46508300
; 48416651
; 49983793
; 57364037
; 78587898
; 90340949
; 93166962
; 93619661
; 103558689
; 113992926
; 118048541
; 121361634
; 124893803
; 126662049
; 131328953
; 134223023
; 134337874
; 135060465
; 135569875
; 135723472
; 136043950
; 137002500
; 139097173
; 144205006
; 144206703
; 151982735
; 152035469
; 160848877
; 160963864
; 162176909
; 163122933
; 163564878
; 164813794
; 172919751
; 175266111
; 175612605
; 178103069
; 179116928
; 184545941
; 210279756
; 210282079
; 223702475
; 224010570
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0M5OC
- Formula
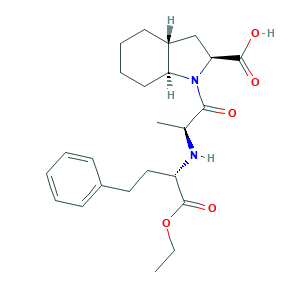
- C24H34N2O5
- Canonical SMILES
- CCOC(=O)C(CCC1=CC=CC=C1)NC(C)C(=O)N2C3CCCCC3CC2C(=O)O
- InChI
- 1S/C24H34N2O5/c1-3-31-24(30)19(14-13-17-9-5-4-6-10-17)25-16(2)22(27)26-20-12-8-7-11-18(20)15-21(26)23(28)29/h4-6,9-10,16,18-21,25H,3,7-8,11-15H2,1-2H3,(H,28,29)/t16-,18+,19-,20-,21-/m0/s1
- InChIKey
- VXFJYXUZANRPDJ-WTNASJBWSA-N
|