Details of the Drug Metabolized by Drug-Metabolizing Enzyme (DME)
| General Information of Drug (ID: DR3541) | ||||||
|---|---|---|---|---|---|---|
| Drug Name |
TAS-120
|
|||||
| Synonyms | . | |||||
| Indication | Hepatocellular carcinoma [ICD11: 2C12] | Phase 3 | [1] | |||
| Multiple myeloma [ICD11: ICD11: 2A83] | Phase 1/2 | [2] | ||||
| Solid tumour/cancer [ICD11: ICD11: 2A00-2F9Z] | Phase 1/2 | [3] | ||||
| Structure |
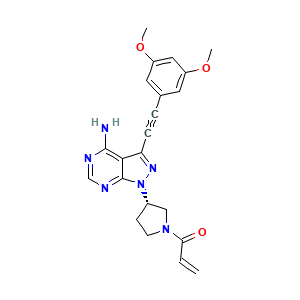 |
|||||
| 3D MOL | 2D MOL | |||||
| Pharmaceutical Properties | Molecular Weight | 418.4 | Topological Polar Surface Area | 108 | ||
| Heavy Atom Count | 31 | Rotatable Bond Count | 6 | |||
| Hydrogen Bond Donor Count | 1 | Hydrogen Bond Acceptor Count | 7 | |||
| Cross-matching ID |
|
|||||
| The Metabolic Roadmap of This Drug | |||||
|---|---|---|---|---|---|
| The Full List of Drug Metabolites (DM) of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | ||||||
|---|---|---|---|---|---|---|
| 1 | ClinicalTrials.gov (NCT04093362) Futibatinib Versus Gemcitabine-Cisplatin Chemotherapy as First-Line Treatment of Patients With Advanced Cholangiocarcinoma Harboring FGFR2 Gene Rearrangements (FOENIX-CCA3). U.S. National Institutes of Health. | |||||
| 2 | ClinicalTrials.gov (NCT02052778) A Dose Finding Study Followed by a Safety and Efficacy Study in Patients With Advanced Solid Tumors or Multiple Myeloma With FGF/FGFR-Related Abnormalities. U.S. National Institutes of Health. | |||||
| 3 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| 4 | Evaluation of the Mass Balance and Metabolic Profile of Futibatinib in Healthy Participants | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.

