| General Information of Drug (ID:
DR0031) |
| Drug Name |
Acalabrutinib
|
| Synonyms |
Acalabrutinib; Acalabrutinib (ACP-196); Acalabrutinib (JAN/USAN/INN); Acalabrutinib [INN]; Acalabrutinib [USAN:INN]; Acalabrutinib(ACP196); Benzamide, 4-(8-amino-3-((2S)-1-(1-oxo-2-butyn-1-yl)-2-pyrrolidinyl)imidazo(1,5-a)pyrazin-1-yl)-N-2-pyridinyl-; Benzamide, 4-[8-amino-3-[(2S)-1-(1-oxo-2-butyn-1-yl)-2-pyrrolidinyl]imidazo[1,5-a]pyrazin-1-yl]-N-2-pyridinyl-; CHEMBL3707348; EX-A881; GTPL8912; I42748ELQW; SCHEMBL14637368; UNII-I42748ELQW; ACP 196; Calquence; Calquence (TN); 1420477-60-6; ACP-196
|
| Indication |
Mantle cell lymphoma
[ICD11: 2A85]
|
Approved
|
[1]
|
| Structure |
|
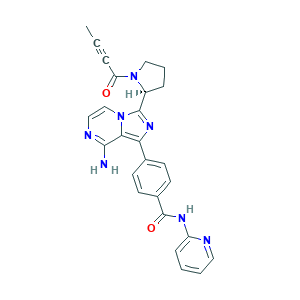 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
465.5 |
Topological Polar Surface Area |
119 |
| Heavy Atom Count |
35 |
Rotatable Bond Count |
4 |
| Hydrogen Bond Donor Count |
2 |
Hydrogen Bond Acceptor Count |
6 |
| Cross-matching ID |
- PubChem CID
- 71226662
- CAS Number
-
- TTD Drug ID
- D09PQZ
- Formula
- C26H23N7O2
- Canonical SMILES
- CC#CC(=O)N1CCCC1C2=NC(=C3N2C=CN=C3N)C4=CC=C(C=C4)C(=O)NC5=CC=CC=N5
- InChI
- 1S/C26H23N7O2/c1-2-6-21(34)32-15-5-7-19(32)25-31-22(23-24(27)29-14-16-33(23)25)17-9-11-18(12-10-17)26(35)30-20-8-3-4-13-28-20/h3-4,8-14,16,19H,5,7,15H2,1H3,(H2,27,29)(H,28,30,35)/t19-/m0/s1
- InChIKey
- WDENQIQQYWYTPO-IBGZPJMESA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


