| Synonyms |
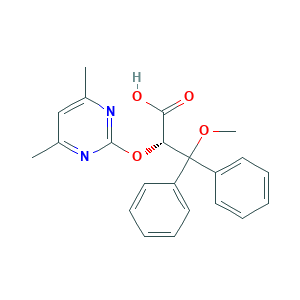
Ambrisentan; BSF 208075; BSF-208075; LU-208075; LU208075; Letairis; Volibris; HW6NV07QEC; LU 208075; (2S)-2-(4,6-dimethylpyrimidin-2-yl)oxy-3-methoxy-3,3-diphenylpropanoic acid; (S)-2-((4,6-Dimethylpyrimidin-2-yl)oxy)-3-methoxy-3,3-diphenylpropanoic acid; (S)-2-(4,6-Dimethylpyrimidin-2-yloxy)-3-methoxy-3,3-diphenylpropanoic acid; (s)-2-[(4,6-dimethylpyrimidin-2-yl)oxy]-3-methoxy-3,3-diphenylpropionic acid; 177036-94-1; CAS-177036-94-1; CHEMBL1111; DSSTox_CID_26282; DSSTox_GSID_46282; DSSTox_RID_81508; UNII-HW6NV07QEC
|
| Cross-matching ID |
- PubChem CID
- 6918493
- PubChem SID
-
12015371
; 14779987
; 17194924
; 43529863
; 50551569
; 51091419
; 53787770
; 57371956
; 93309673
; 103307060
; 104035299
; 114788055
; 118855302
; 126616865
; 126651844
; 131302629
; 134338642
; 135692418
; 137237404
; 139988399
; 143497715
; 144205520
; 152133959
; 152238501
; 152258462
; 160647297
; 160826914
; 162011508
; 162172038
; 163093066
; 163387054
; 164824132
; 170465143
; 174007223
; 175267375
; 175426501
; 178100778
; 179116973
; 185986582
; 187071933
; 196107821
; 198977886
; 198991664
; 204430246
; 211534802
; 223375980
; 223532574
; 223704800
; 224518792
; 226395806
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0X5ZI
- Formula
- C22H22N2O4
- Canonical SMILES
- CC1=CC(=NC(=N1)OC(C(=O)O)C(C2=CC=CC=C2)(C3=CC=CC=C3)OC)C
- InChI
- 1S/C22H22N2O4/c1-15-14-16(2)24-21(23-15)28-19(20(25)26)22(27-3,17-10-6-4-7-11-17)18-12-8-5-9-13-18/h4-14,19H,1-3H3,(H,25,26)/t19-/m1/s1
- InChIKey
- OUJTZYPIHDYQMC-LJQANCHMSA-N
|