| Synonyms |
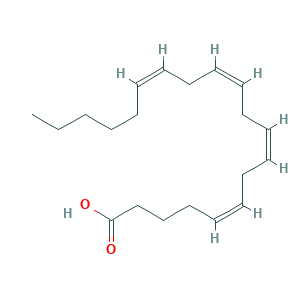
Arachidonic Acid, 99%; Immunocytophyte; YZXBAPSDXZZRGB-DOFZRALJSA-N; arachidonate; arachidonic acid; (5Z,8Z,11Z,14Z)-icosa-5,8,11,14-tetraenoic acid; (all-Z)-5,8,11,14-Eicosatetraenoic acid; 27YG812J1I; 5,8,11,14-Eicosatetraenoic acid; 5,8,11,14-Eicosatetraenoic acid, (all-Z)-; 506-32-1; 5Z,8Z,11Z,14Z-eicosatetraenoic acid; CHEBI:15843; CHEMBL15594; MFCD00004417; UNII-27YG812J1I; all-cis-5,8,11,14-eicosatetraenoic acid; cis,cis,cis,cis-5,8,11,14-Eicosatetraenoic acid; cis-5,8,11,14-Eicosatetraenoic acid
|
| Cross-matching ID |
- PubChem CID
- 444899
- PubChem SID
-
3519
; 584562
; 619099
; 3139094
; 4265935
; 7885669
; 7979717
; 8145109
; 10299602
; 10524489
; 14800661
; 24846907
; 24890733
; 24890784
; 24891487
; 26747902
; 26753719
; 26753720
; 26753721
; 26758248
; 36887771
; 46391417
; 46392023
; 46393430
; 47885166
; 47959472
; 48110218
; 48493842
; 49681170
; 49846134
; 49846384
; 50107316
; 50326117
; 53787885
; 56311297
; 56311317
; 56312730
; 56312894
; 56313841
; 56313927
; 56365480
; 57261284
; 57404647
; 57646859
; 85788730
; 87562313
; 91011640
; 92298150
; 92309955
; 92713311
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0HD9P
- Formula
- C20H32O2
- Canonical SMILES
- CCCCCC=CCC=CCC=CCC=CCCCC(=O)O
- InChI
- 1S/C20H32O2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20(21)22/h6-7,9-10,12-13,15-16H,2-5,8,11,14,17-19H2,1H3,(H,21,22)/b7-6-,10-9-,13-12-,16-15-
- InChIKey
- YZXBAPSDXZZRGB-DOFZRALJSA-N
|