| General Information of Drug (ID:
DR0210) |
| Drug Name |
Bicalutamide
|
| Synonyms |
Bicalutamide (CDX); Bicalutamide (Casodex); Bicalutamide(Casodex); Calutide; Casodex; Cosudex; Kalumid; LKJPYSCBVHEWIU-UHFFFAOYSA-N; bicalutamide; (+-)-4'-Cyano-alpha,alpha,alpha-trifluoro-3-((p-fluorophenyl)sulfonyl)-2-methyl-m-lactotoluidide; 90357-06-5; BRN 5364666; C18H14F4N2O4S; CHEMBL409; ICI 176334; ICI-176334; MFCD00869971; N-[4-Cyano-3-(trifluoromethyl)phenyl]-3-[(4-fluorophenyl)sulfonyl]-2-hydroxy-2-methylpropanamide; N-[4-cyano-3-(trifluoromethyl)phenyl]-3-(4-fluorophenyl)sulfonyl-2-hydroxy-2-methylpropanamide
|
| Indication |
Prostate cancer
[ICD11: 2C82]
|
Approved
|
[1]
|
| Structure |
|
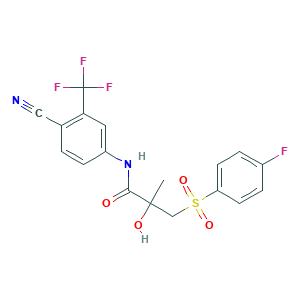 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
430.4 |
Topological Polar Surface Area |
116 |
| Heavy Atom Count |
29 |
Rotatable Bond Count |
5 |
| Hydrogen Bond Donor Count |
2 |
Hydrogen Bond Acceptor Count |
9 |
| Cross-matching ID |
- PubChem CID
- 2375
- PubChem SID
-
10360
; 536636
; 4422035
; 7848024
; 7978493
; 8151593
; 11528703
; 12014029
; 14807515
; 26719836
; 29221542
; 46386630
; 46505386
; 46518598
; 49681685
; 49835627
; 50113021
; 50709017
; 53790604
; 57321290
; 85174226
; 85174230
; 90341766
; 91610973
; 92308263
; 92308948
; 92711409
; 93815120
; 99016301
; 99437214
; 103164349
; 104300407
; 118046574
; 121362017
; 124658945
; 124757067
; 124800143
; 124893438
; 125163871
; 125338410
; 125359510
; 125545351
; 126630887
; 126657361
; 126665838
; 128501765
; 131295154
; 134358448
; 135014383
; 135649993
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0V9BD
- Formula
- C18H14F4N2O4S
- Canonical SMILES
- CC(CS(=O)(=O)C1=CC=C(C=C1)F)(C(=O)NC2=CC(=C(C=C2)C#N)C(F)(F)F)O
- InChI
- 1S/C18H14F4N2O4S/c1-17(26,10-29(27,28)14-6-3-12(19)4-7-14)16(25)24-13-5-2-11(9-23)15(8-13)18(20,21)22/h2-8,26H,10H2,1H3,(H,24,25)
- InChIKey
- LKJPYSCBVHEWIU-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


