| General Information of Drug (ID:
DR0347) |
| Drug Name |
Clofibrate
|
| Synonyms |
Chlorphenisate; Citiflus; Claripex; Clofibate; Clofibrato; Clofibratum; Clofinit; Amotril; Amotril S; Angiokapsul; Anparton; Antilipid; Antilipide; Apolan; Arterioflexin; Arterosol; Ateculon; Ateriosan; Athebrate; Atheromide; Atheropront; Atrolen; Atromid; Atromid-S; Atromida; Bioscleran; Ethyl chlorophenoxyisobutyrate; Ethyl clofibrate; Fibralem; Klofiran; Lipavil; Lipavlon; Lipofacton; Lipomid; Liprin; Liprinal; Lobetrin; Miscleron; Regardin; Serotinex; Ticlobran; Xyduril; clofibrate; 637-07-0; EPIB; Ethyl 2-(4-chlorophenoxy)-2-methylpropanoate
|
| Indication |
Hypertriglyceridaemia
[ICD11: 5C80]
|
Approved
|
[1]
|
| Structure |
|
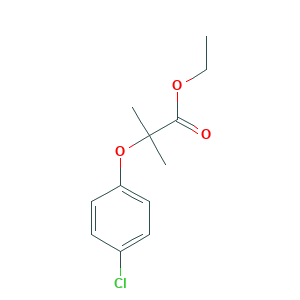 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
242.7 |
Topological Polar Surface Area |
35.5 |
| Heavy Atom Count |
16 |
Rotatable Bond Count |
5 |
| Hydrogen Bond Donor Count |
0 |
Hydrogen Bond Acceptor Count |
3 |
| Cross-matching ID |
- PubChem CID
- 2796
- PubChem SID
-
9133
; 119396
; 221613
; 893268
; 3218686
; 4302581
; 7847345
; 7978969
; 8147031
; 8149997
; 8150180
; 8151805
; 10531332
; 10852042
; 11110971
; 11120027
; 11120515
; 11121003
; 11121642
; 11122122
; 11147110
; 11335997
; 11336101
; 11361236
; 11361340
; 11362711
; 11364629
; 11365273
; 11367191
; 11367835
; 11369753
; 11370693
; 11370694
; 11373436
; 11374024
; 11375997
; 11377915
; 11446100
; 11452212
; 11462208
; 11462312
; 11484734
; 11489025
; 11492038
; 11495549
; 14798427
; 17404869
; 22391427
; 22395216
; 24278085
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0J5DC
- Formula
- C12H15ClO3
- Canonical SMILES
- CCOC(=O)C(C)(C)OC1=CC=C(C=C1)Cl
- InChI
- 1S/C12H15ClO3/c1-4-15-11(14)12(2,3)16-10-7-5-9(13)6-8-10/h5-8H,4H2,1-3H3
- InChIKey
- KNHUKKLJHYUCFP-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


