| Synonyms |
Dolutegravir (sodium); Dolutegravir Sodium ( API); GSK 1349572A; GSK-1349572A; GSK1349572 sodiuM salt; GSK1349572A; Sodium (4R,12aS)-9-((2,4-difluorobenzyl)carbamoyl)-4-methyl-6,8-dioxo-3,4,6,8,12,12a-hexahydro-2H-pyrido[1',2':4,5]pyrazino[2,1-b][1,3]oxazin-7-olate;Sodium (4R,12aS)-9-((2,4-difluorobenzyl)carbamoyl)-4-methyl-6,8-dioxo-3,4,6,8,12,12a-hexahydro-2H-pyrido[1',2':4,5]pyrazino[2,1-b][1,3]oxazin-7-olate; UNII-1Q1V9V5WYQ; 1051375-19-9; 1Q1V9V5WYQ; CHEBI:76007; DOLUTEGRAVIR SODIUM
|
| Cross-matching ID |
- PubChem CID
- 46216142
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D00YZD
- Formula
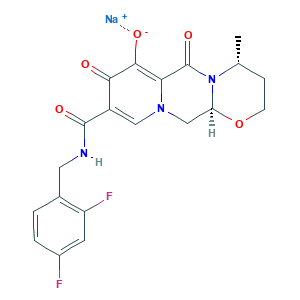
- C20H18F2N3NaO5
- Canonical SMILES
- CC1CCOC2N1C(=O)C3=C(C(=O)C(=CN3C2)C(=O)NCC4=C(C=C(C=C4)F)F)[O-].[Na+]
- InChI
- 1S/C20H19F2N3O5.Na/c1-10-4-5-30-15-9-24-8-13(17(26)18(27)16(24)20(29)25(10)15)19(28)23-7-11-2-3-12(21)6-14(11)22;/h2-3,6,8,10,15,27H,4-5,7,9H2,1H3,(H,23,28);/q;+1/p-1/t10-,15+;/m1./s1
- InChIKey
- UGWJRRXTMKRYNK-VSLILLSYSA-M
|