| Synonyms |
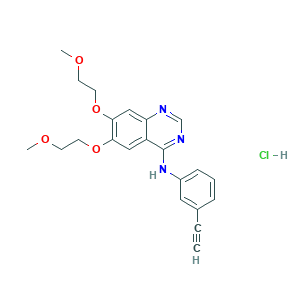
Erlotinib (Hydrochloride); Erlotinib HCl (OSI-744); Erlotinib hydrochloride; Erlotinib; J4T82NDH7E; N-(3-Ethynylphenyl)-6,7-bis(2-methoxyethoxy)-4-quinazolinamine; N-(3-Ethynylphenyl)-6,7-bis(2-methoxyethoxy)quinazolin-4-amine; NCGC00164574-01; UNII-J4T82NDH7E; [6,7-BIS(2-METHOXY-ETHOXY)QUINAZOLINE-4-YL]-(3-ETHYNYLPHENYL)AMINE; 183321-74-6; 4-Quinazolinamine, N-(3-ethynylphenyl)-6,7-bis(2-methoxyethoxy)-; 4-[(3-Ethynylphenyl)amino]-6,7-bis(2-methoxyethoxy)quinazoline; AK142763; CHEBI:114785; CHEMBL553; CP358774; DSSTox_CID_26454; DSSTox_GSID_46454; DSSTox_RID_81628; MFCD07781272; N-(3-ethynylphenyl)-6,7-bis(2-methoxyethoxy)quinazolin-4-amine hydrochloride; NSC-718781; OSI 774; Tarceva; UNII-DA87705X9K; erlotinib HCl; (CP358774; 183319-69-9; 4-Quinazolinamine, N-(3-ethynylphenyl)-6,7-bis(2-methoxyethoxy)-, hydrochloride (1:1); 6,7-bis(2-methoxyethoxy)-4-(3-ethynylanilino)quinazoline hcl; 6,7-bis(2-methoxyethoxy)-4-(3-ethynylanilino)quinazoline hydrochloride; CP 358774; CP-358774; DA87705X9K
|
| Cross-matching ID |
- PubChem CID
- 176871
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D07POC
- Formula
- C22H24ClN3O4
- Canonical SMILES
- COCCOC1=C(C=C2C(=C1)C(=NC=N2)NC3=CC=CC(=C3)C#C)OCCOC
- InChI
- 1S/C22H23N3O4/c1-4-16-6-5-7-17(12-16)25-22-18-13-20(28-10-8-26-2)21(29-11-9-27-3)14-19(18)23-15-24-22/h1,5-7,12-15H,8-11H2,2-3H3,(H,23,24,25)
- InChIKey
- GTTBEUCJPZQMDZ-UHFFFAOYSA-N
|