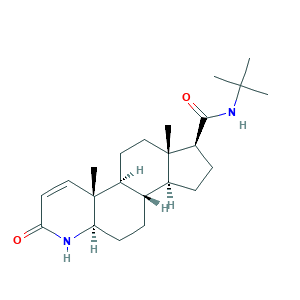
| Synonyms |
Finasterida; Finasterida [INN-Spanish]; Finasteridum; Finasteridum [INN-Latin]; Finastid; Finpecia; L-652,931; MK 0906; MK 906; Chibro-Proscar; MK-0906; MK-906; Propecia; Proscar; Prostide; finasteride; (5alpha,17beta)-(1,1-Dimethylethyl)-3-oxo-4-azaandrost-1-ene-17-carboxamide; 57GNO57U7G; 98319-26-7; BRN 4269024; CCRIS 7438; CHEBI:5062; CHEMBL710; HSDB 6793; N-tert-Butyl-3-oxo-4-aza-5alpha-androst-1-ene-17beta-carboxamide; UNII-57GNO57U7G
|
| Cross-matching ID |
- PubChem CID
- 57363
- PubChem SID
-
7847387
; 7979227
; 8185196
; 11466745
; 11467865
; 11486590
; 11528597
; 11533303
; 12014292
; 12146021
; 14779683
; 14926633
; 24724479
; 24861022
; 25819911
; 26719811
; 43115066
; 46386559
; 46507645
; 47424400
; 47646719
; 48094722
; 48394119
; 48413780
; 48416011
; 49666458
; 49681675
; 49699149
; 53790828
; 56313630
; 57313897
; 57649138
; 74382929
; 75258634
; 85788501
; 91146246
; 92125899
; 92308339
; 92309138
; 99437022
; 99444245
; 103194727
; 103914483
; 103975037
; 104312468
; 117571916
; 118048887
; 121363570
; 124658933
; 124757073
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D08IWD
- Formula
- C23H36N2O2
- Canonical SMILES
- CC12CCC3C(C1CCC2C(=O)NC(C)(C)C)CCC4C3(C=CC(=O)N4)C
- InChI
- 1S/C23H36N2O2/c1-21(2,3)25-20(27)17-8-7-15-14-6-9-18-23(5,13-11-19(26)24-18)16(14)10-12-22(15,17)4/h11,13-18H,6-10,12H2,1-5H3,(H,24,26)(H,25,27)/t14-,15-,16-,17+,18+,22-,23+/m0/s1
- InChIKey
- DBEPLOCGEIEOCV-WSBQPABSSA-N
|