| General Information of Drug (ID:
DR0788) |
| Drug Name |
Granisetron hydrochloride
|
| Synonyms |
Granisetronum; Granisetron Hydrocholride; Granisetron hydrochloride; Granisetron-MDTS; Granisol; BEMA-Granisetron; Kytril; Kytril Injection; 1-METHYL-1H-INDAZOLE-3-CARBOXYLIC ACID (9-METHYL-9-AZA-BICYCLO[3.3.1]NON-3-YL)-AMIDE HYDROCHLORIDE; 1-Methyl-N-[(3-endo)-9-methyl-9-azabicyclo[3.3.1]non-3-yl]-1H-indazole-3-carboxamide hydrochloride; 1-methyl-N-[(1S,5R)-9-methyl-9-azabicyclo[3.3.1]nonan-3-yl]indazole-3-carboxamide hydrochloride; 107007-99-8; 318F6L70J8; BRL 43694A; MFCD01747034; NCGC00183873-01; UNII-318F6L70J8; Kevatril; Sustol; WZG3J2MCOL; granisetron; 1-Methyl-N-(9-methyl-endo-9-azabicyclo(3.3.1)non-3-yl)-1H-indazole-3-carboxamide; 1-Methyl-N-[(1r,5s)-9-Methyl-9-Azabicyclo[3.3.1]nonan-3-Yl]indazole-3-Carboxamide; 1-methyl-N-[(1S,5R)-9-methyl-9-azabicyclo[3.3.1]nonan-3-yl]indazole-3-carboxamide; 1-methyl-N-[(3-endo)-9-methyl-9-azabicyclo[3.3.1]non-3-yl]-1H-indazole-3-carboxamide; 109889-09-0; AC1NR4P1; BRL 43694; BRL 43964; BRL-43694; CHEBI:5537; CHEMBL1290003; UNII-WZG3J2MCOL; APF 530; APF530
|
| Indication |
Functional nausea/vomiting
[ICD11: DD90]
|
Approved
|
[1]
|
| Structure |
|
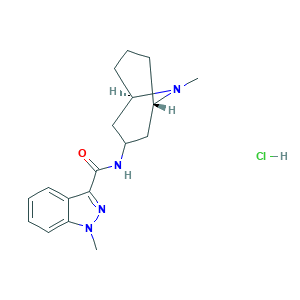 |
|
3D MOL is unavailable
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
348.9 |
Topological Polar Surface Area |
50.2 |
| Heavy Atom Count |
24 |
Rotatable Bond Count |
2 |
| Hydrogen Bond Donor Count |
2 |
Hydrogen Bond Acceptor Count |
3 |
| Cross-matching ID |
- PubChem CID
- 6918003
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0J5KF
- Formula
- C18H25ClN4O
- Canonical SMILES
- CN1C2CCCC1CC(C2)NC(=O)C3=NN(C4=CC=CC=C43)C.Cl
- InChI
- 1S/C18H24N4O.ClH/c1-21-13-6-5-7-14(21)11-12(10-13)19-18(23)17-15-8-3-4-9-16(15)22(2)20-17;/h3-4,8-9,12-14H,5-7,10-11H2,1-2H3,(H,19,23);1H/t12?,13-,14+;
- InChIKey
- QYZRTBKYBJRGJB-PCMHIUKPSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


