Details of the Drug Metabolized by Drug-Metabolizing Enzyme (DME)
| General Information of Drug (ID: DR0907) | ||||||
|---|---|---|---|---|---|---|
| Drug Name |
Ketoprofen
|
|||||
| Synonyms |
Kefenid; Ketoprofene; Ketoprofeno; Ketoprofenum [INN-Latin]; Ketopron; Ketoprophene; Lertus; Menamin; Meprofen; Orudis; Orudis (TN); Orudis KT; Orugesic; Oruvail; Oscorel; Profenid; RP 19583; RP-19583; Toprec; Toprek; ketoprofen; m-Benzoylhydratropic acid; Actron; Alrheumat; Alrheumun; Capisten; Epatec; Fastum; racemic-Ketoprofen; 2-(3-Benzoylphenyl)propanoic acid; 2-(3-Benzoylphenyl)propionic acid; 2-(m-Benzoylphenyl)propionic acid; 2-[3-(phenylcarbonyl)phenyl]propanoic acid; 22071-15-4; 3-Benzoylhydratropic acid; Aneol; Dexal; Iso-K
|
|||||
| Indication | Anaesthesia [ICD11: 8E22] | Approved | [1] | |||
| Structure |
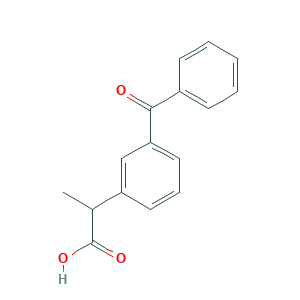 |
|||||
| 3D MOL | 2D MOL | |||||
| Pharmaceutical Properties | Molecular Weight | 254.28 | Topological Polar Surface Area | 54.4 | ||
| Heavy Atom Count | 19 | Rotatable Bond Count | 4 | |||
| Hydrogen Bond Donor Count | 1 | Hydrogen Bond Acceptor Count | 3 | |||
| Cross-matching ID |
|
|||||
| The Metabolic Roadmap of This Drug | |||||
|---|---|---|---|---|---|
| The Full List of Drug Metabolites (DM) of This Drug | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug-Metabolizing Enzyme(s) (DME) Metabolizing This Drug | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||
| References | ||||||
|---|---|---|---|---|---|---|
| 1 | Ketoprofen was approved by FDA. The 2020 official website of the U.S. Food and Drug Administration. | |||||
| 2 | Clinical pharmacokinetics of ketoprofen enantiomers in wild type of Cyp 2c8 and Cyp 2c9 patients with rheumatoid arthritis Eur J Drug Metab Pharmacokinet. 2011 Sep;36(3):167-73. doi: 10.1007/s13318-011-0041-1. | |||||
| 3 | Clinical pharmacokinetics of ketoprofen enantiomers in wild type of Cyp 2c8 and Cyp 2c9 patients with rheumatoid arthritis. Eur J Drug Metab Pharmacokinet. 2011 Sep;36(3):167-73. | |||||
| 4 | Association between the UGT1A1*28 allele and hyperbilirubinemia in HIV-positive patients receiving atazanavir: a meta-analysis. Biosci Rep. 2019 May 2;39(5). pii: BSR20182105. | |||||
| 5 | Characterization and quantification of metabolites of racemic ketoprofen excreted in urine following oral administration to man by 1H-NMR spectroscopy, directly coupled HPLC-MS and HPLC-NMR, and circular dichroism Xenobiotica. 2004 Nov-Dec;34(11-12):1075-89. doi: 10.1080/00498250412331281098. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.

