Details of the Drug Metabolized by Drug-Metabolizing Enzyme (DME)
| General Information of Drug (ID: DR1048) | ||||||
|---|---|---|---|---|---|---|
| Drug Name |
Methoxyflurane
|
|||||
| Synonyms |
Methofane; Methoflurane; Methoxane; Methoxiflurane; Methoxifluranum; Methoxyfluoran; Methoxyfluorane; Methoxyfluran; Methoxyfluranum; Methoxyfluranum [INN-Latin]; Metofane; Metofane (VAN); Metossiflurano [DCIT]; Metoxfluran; Metoxifluran; Metoxiflurano; Metoxiflurano [INN-Spanish]; Penthrane; Penthrane (VAN); Pentran; Pentrane; methoxyflurane; Analgizer; Anecotan; Ingalan; Inhalan; 2,2-Dichloro-1,1-difluoro-1-methoxyethane; 2,2-Dichloro-1,1-difluoroethyl methyl ether; 76-38-0; Ethane, 2,2-dichloro-1,1-difluoro-1-methoxy-
|
|||||
| Indication | Anaesthesia [ICD11: 8E22] | Approved | [1] | |||
| Structure |
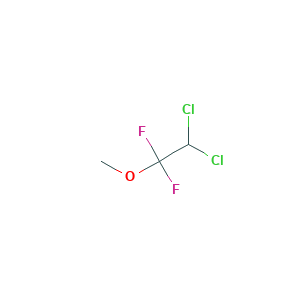 |
|||||
| 3D MOL | 2D MOL | |||||
| Pharmaceutical Properties | Molecular Weight | 164.96 | Topological Polar Surface Area | 9.2 | ||
| Heavy Atom Count | 8 | Rotatable Bond Count | 2 | |||
| Hydrogen Bond Donor Count | 0 | Hydrogen Bond Acceptor Count | 3 | |||
| Cross-matching ID |
|
|||||
| Drug-Metabolizing Enzyme(s) (DME) Metabolizing This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.

