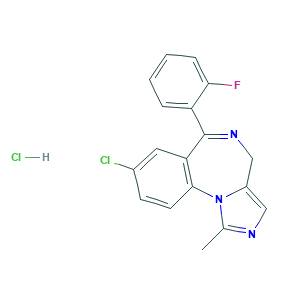
| Synonyms |
Midazolam Base; Midazolamum; Midazolamum [INN-Latin]; Midazolam Hcl; Midazolam hydrochloride (USAN); Midazolam hydrochloride [USAN]; Ro 21-3981/001; Ro 21-3981/003; W7TTW573JJ; 59467-96-8; 8-Chloro-6-(2-fluorophenyl)-1-methyl-4H-imidazo(1,5-a)(1,4)benzodiazepine hydrochloride; 8-chloro-6-(2-fluorophenyl)-1-methyl-4H-imidazo[1,5-a][1,4]benzodiazepine hydrochloride; DSSTox_CID_27824; DSSTox_GSID_47846; DSSTox_RID_82587; EINECS 261-776-6; HSDB 6751; Rocam; UNII-W7TTW573JJ; Hypnovel; MIDAZOLAM HYDROCHLORIDE; R60L0SM5BC; Ro 21-3981; mezolam; midazolam; 59467-70-8; 8-Chlor-6-(2-fluorphenyl)-1-methyl-4H-imidazo(1,5-a)(1,4)benzodiazepin; 8-Chloro-6-(2-fluorophenyl)-1-methyl-4H-imidazo[1,5-a][1,4]benzodiazepine; 8-Chloro-6-(o-fluorophenyl)-1-methyl-4H-imidazo(1,5-a)(1,4)benzodiazepine; BRN 0625572; C18H13ClFN3; CHEBI:6931; CHEMBL655; DEA No. 2884; EINECS 261-774-5; UNII-R60L0SM5BC; Buccolam; DDLIGBOFAVUZHB-UHFFFAOYSA-N
|