| Synonyms |
Ondansetron [INN]; Ondansetron [USAN]; SN-307; Zofran; Zofran ODT; Zophren; Zuplenz; ondansetron; ondansetron (Zofran); 1,2,3,9-Tetrahydro-9-methyl-3-((2-methyl-1H-imidazol-1-yl)methyl)-4H-carbazol-4-one; 116002-70-1; 9-Methyl-3-((2-methyl-1H-imidazol-1-yl)methyl)-2,3-dihydro-1H-carbazol-4(9H)-one; 9-methyl-3-[(2-methyl-1H-imidazol-1-yl)methyl]-2,3,4,9-tetrahydro-1H-carbazol-4-one; 9-methyl-3-[(2-methylimidazol-1-yl)methyl]-2,3-dihydro-1H-carbazol-4-one; 99614-02-5; BRN 3622981; C18H19N3O; CHEBI:7773; GR 38032; GR 38032X; GR-38032F; GR38032; NCGC00179341-02; Zudan
|
| Cross-matching ID |
- PubChem CID
- 4595
- PubChem SID
-
9533
; 3206266
; 3966923
; 4696243
; 7847522
; 7980201
; 8152820
; 11137931
; 11427135
; 11467086
; 11468206
; 11486674
; 11501532
; 14873468
; 29223684
; 46504819
; 47389509
; 47686452
; 47686453
; 47909550
; 47909551
; 47984334
; 48416352
; 49698991
; 49835876
; 49901808
; 50064204
; 50949003
; 53787007
; 57322341
; 58007430
; 85174434
; 85209216
; 85787808
; 85788905
; 92309152
; 93624973
; 103166621
; 103915041
; 104170204
; 104225172
; 104306859
; 105146690
; 121362488
; 124579243
; 124893606
; 125324981
; 125360632
; 125814185
; 126525328
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0K7WK
- Formula
- C18H19N3O
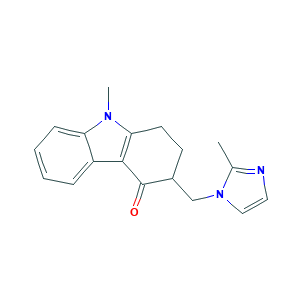
- Canonical SMILES
- CC1=NC=CN1CC2CCC3=C(C2=O)C4=CC=CC=C4N3C
- InChI
- 1S/C18H19N3O/c1-12-19-9-10-21(12)11-13-7-8-16-17(18(13)22)14-5-3-4-6-15(14)20(16)2/h3-6,9-10,13H,7-8,11H2,1-2H3
- InChIKey
- FELGMEQIXOGIFQ-UHFFFAOYSA-N
|