| General Information of Drug (ID:
DR1347) |
| Drug Name |
Proguanil
|
| Prodrug Info |
Proguanil is the prodrug of Cycloguanil
|
| Synonyms |
Paludrin; Paludrine; Proguanil [INN:BAN]; Proguanile; Proguanile [DCIT]; Proguanilum; Proguanilum [INN-Latin]; S61K3P7B2V; Bigumal; Chlorguanid; Chlorguanide; Chloriguane; Chloroguanide; proguanil; 1-(p-Chlorophenyl)-5-isopropylbiguanide; 1-Isopropyl-5-(4-chlorophenyl)biguanide; 500-92-5; BIGUANIDE, 1-(p-CHLOROPHENYL)-5-ISOPROPYL-; BRN 2811599; C11H16ClN5; CHEBI:8455; EINECS 207-915-6; Imidodicarbonimidic diamide, N-(4-chlorophenyl)-N'-(1-methylethyl)-; N1-p-Chlorophenyl-N5-isopropylbiguanide; UNII-S61K3P7B2V
|
| Indication |
Malaria
[ICD11: 1F40]
|
Phase 4
|
[1]
|
| Structure |
|
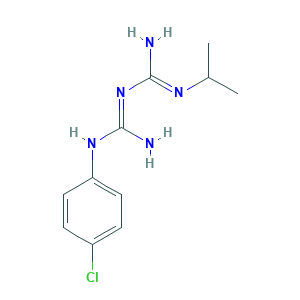 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
253.73 |
Topological Polar Surface Area |
88.8 |
| Heavy Atom Count |
17 |
Rotatable Bond Count |
4 |
| Hydrogen Bond Donor Count |
3 |
Hydrogen Bond Acceptor Count |
1 |
| Cross-matching ID |
- PubChem CID
- 6178111
- PubChem SID
-
9833
; 603149
; 7980396
; 11112433
; 11341938
; 11362121
; 11364482
; 11367044
; 11369606
; 11372736
; 11374343
; 11377768
; 11467027
; 11468147
; 11484765
; 11486724
; 11487523
; 11488951
; 11491474
; 11492437
; 11495402
; 14823534
; 47736673
; 47885583
; 47959951
; 48035321
; 48035322
; 48185162
; 48415754
; 49698968
; 49893807
; 50100438
; 50100439
; 57309709
; 57365545
; 80043130
; 85788592
; 87246413
; 93166734
; 96025114
; 103462237
; 114370477
; 134978163
; 137004047
; 137040200
; 137131382
; 160964465
; 162188072
; 175267378
; 179039118
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0P8RS
- Formula
- C11H16ClN5
- Canonical SMILES
- CC(C)N=C(N)N=C(N)NC1=CC=C(C=C1)Cl
- InChI
- 1S/C11H16ClN5/c1-7(2)15-10(13)17-11(14)16-9-5-3-8(12)4-6-9/h3-7H,1-2H3,(H5,13,14,15,16,17)
- InChIKey
- SSOLNOMRVKKSON-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


