Details of the Drug Metabolized by Drug-Metabolizing Enzyme (DME)
| General Information of Drug (ID: DR1420) | ||||||
|---|---|---|---|---|---|---|
| Drug Name |
Rifampicin
|
|||||
| Synonyms |
Rifadin; Rifadine; Rifagen; Rifaldazin; Rifaldazine; Rifaldin; Rifamor; Rifampicin; Rifampicin SV; Rifampicina; Rifampicina [INN-Spanish]; Rifampicine [French]; Rifampicinum; Rifampicinum [INN-Latin]; Rifamycin AMP; Rifaprodin; Rifcin; Rifinah; Rifobac; Rifoldin; Rifoldine; Riforal; Rimactan; Rimactane; Rimactazid; Rimactizid; Rimazid; Sinerdol; Tubocin; Archidyn; Arficin; Arzide; Benemicin; Dione 21-acetate; Doloresum; Eremfat; Fenampicin; L-5103 Lepetit; rifampin; 13292-46-1; Abrifam; Ba 41166/E; R/AMP; Rifa; Rifam
|
|||||
| Indication | Osteoporosis [ICD11: FB83] | Approved | [1] | |||
| Structure |
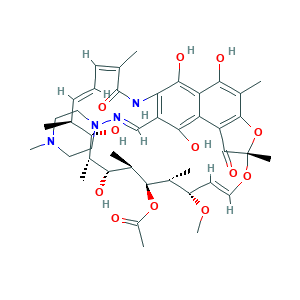 |
|||||
| 3D MOL is unavailable | 2D MOL | |||||
| Pharmaceutical Properties | Molecular Weight | 822.9 | Topological Polar Surface Area | 220 | ||
| Heavy Atom Count | 59 | Rotatable Bond Count | 5 | |||
| Hydrogen Bond Donor Count | 6 | Hydrogen Bond Acceptor Count | 15 | |||
| Cross-matching ID |
|
|||||
| The Metabolic Roadmap of This Drug | |||||
|---|---|---|---|---|---|
| The Full List of Drug Metabolites (DM) of This Drug | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||
| The Full List of Metabolic Reaction (MR) of This Drug | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||
| Drug-Metabolizing Enzyme(s) (DME) Metabolizing This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.

