| Synonyms |
Ropivacaine hydrochloride hydrate; Ropivacaine monohydrochloride; UNII-35504LBE2T; (S)-N-(2,6-dimethylphenyl)-1-propylpiperidine-2-carboxamide hydrochloride; (S)-Ropivacaine hydrochloride; (S)-ropivacaine hydrochloride (anhydrous); Ropivacaina; Ropivacaina [INN-Spanish]; Ropivacaina [Spanish]; Ropivacaine; Ropivacaine (INN); Ropivacaine [INN]; Ropivacainum; Ropivacainum [INN-Latin]; S-Ropivacaine; UNII-7IO5LYA57N; (-)-1-Propyl-2',6'-pipecoloxylidide; (2S)-N-(2,6-dimethylphenyl)-1-propyl-2-piperidinecarboxamide; (2S)-N-(2,6-dimethylphenyl)-1-propylpiperidine-2-carboxamide; (S)-N-(2,6-Dimethylphenyl)-1-propylpiperidine-2-carboxamide; (S)-Ropivacaine; 7IO5LYA57N; 84057-95-4; CHEBI:8890; 132112-35-7; 2-Piperidinecarboxamide, N-(2,6-dimethylphenyl)-1-propyl-, monohydrochloride, (S)-; 35504LBE2T; 98717-15-8; C17H26N2O.HCl.H2O; CAS-98717-15-8; CHEBI:60803; DSSTox_CID_28353; DSSTox_GSID_48379; DSSTox_RID_82753; NCGC00164597-01; ROPIVACAINE HCl
|
| Cross-matching ID |
- PubChem CID
- 175804
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D09RHQ
- Formula
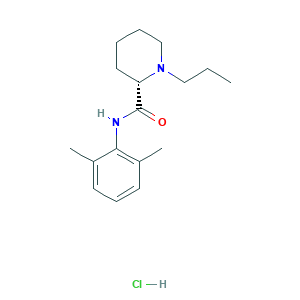
- C17H27ClN2O
- Canonical SMILES
- CCCN1CCCCC1C(=O)NC2=C(C=CC=C2C)C.Cl
- InChI
- 1S/C17H26N2O.ClH/c1-4-11-19-12-6-5-10-15(19)17(20)18-16-13(2)8-7-9-14(16)3;/h7-9,15H,4-6,10-12H2,1-3H3,(H,18,20);1H/t15-;/m0./s1
- InChIKey
- NDNSIBYYUOEUSV-RSAXXLAASA-N
|