| General Information of Drug (ID:
DR1666) |
| Drug Name |
Valdecoxib
|
| Synonyms |
Valdecoxib; Valdyn; YM-974; Bextra; LNPDTQAFDNKSHK-UHFFFAOYSA-N; SC 65872; SC-65872; 181695-72-7; 4-(5-Methyl-3-phenyl-4-isoxazolyl)benzenesulfonamide; 4-(5-methyl-3-phenyl-1,2-oxazol-4-yl)benzenesulfonamide; 4-(5-methyl-3-phenyl-4-isoxazolyl) benzenesulfonamide; 4-(5-methyl-3-phenylisoxazol-4-yl)benzenesulfonamide; Benzenesulfonamide, 4-(5-methyl-3-phenyl-4-isoxazolyl)-; CHEBI:63634; CHEMBL865; COX; HSDB 7302; Kudeq; MFCD00950568; NCGC00095129-01; UNII-2919279Q3W; p-(5-Methyl-3-phenyl-4-isoxazolyl)benzenesulfonamide
|
| Indication |
Osteoarthritis
[ICD11: FA00]
|
Approved
|
[1]
|
| Structure |
|
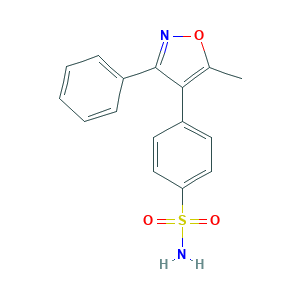 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
314.4 |
Topological Polar Surface Area |
94.6 |
| Heavy Atom Count |
22 |
Rotatable Bond Count |
3 |
| Hydrogen Bond Donor Count |
1 |
Hydrogen Bond Acceptor Count |
5 |
| Cross-matching ID |
- PubChem CID
- 119607
- PubChem SID
-
587378
; 841634
; 7300492
; 7979620
; 10238560
; 11364641
; 11367203
; 11369765
; 11372040
; 11374755
; 11377927
; 11484171
; 11488324
; 11490816
; 11492943
; 11495561
; 11528781
; 11538199
; 12015079
; 14850214
; 17137106
; 17396876
; 26612701
; 26680171
; 26719834
; 26748964
; 26748965
; 29300443
; 46386621
; 46393843
; 46506229
; 46513481
; 47885663
; 48110737
; 49665731
; 49666068
; 49681792
; 50107497
; 53789038
; 57339580
; 57578333
; 58107175
; 75154431
; 85789263
; 88531863
; 92124765
; 92308537
; 92714410
; 99444047
; 103225393
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0L6DA
- Formula
- C16H14N2O3S
- Canonical SMILES
- CC1=C(C(=NO1)C2=CC=CC=C2)C3=CC=C(C=C3)S(=O)(=O)N
- InChI
- 1S/C16H14N2O3S/c1-11-15(12-7-9-14(10-8-12)22(17,19)20)16(18-21-11)13-5-3-2-4-6-13/h2-10H,1H3,(H2,17,19,20)
- InChIKey
- LNPDTQAFDNKSHK-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


