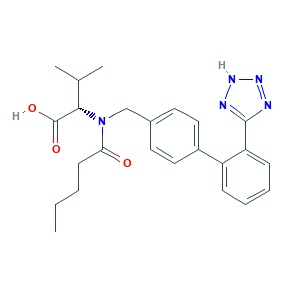
| Synonyms |
Diovan; Exforge; Kalpress; L-Valsartan; Provas; valsartan; (2S)-3-methyl-2-[pentanoyl-[[4-[2-(2H-tetrazol-5-yl)phenyl]phenyl]methyl]amino]butanoic acid; (S)-2-(N-((2'-(1H-Tetrazol-5-yl)-[1,1'-biphenyl]-4-yl)methyl)pentanamido)-3-methylbutanoic acid; 137862-53-4; 137863-60-6; 80M03YXJ7I; C24H29N5O3; CGP 48933; CGP-48933; CHEBI:9927; CHEMBL1069; MFCD00865840; Miten; N-(1-oxopentyl)-N-[[2'-(1H-tetrazol-5-yl)[1,1'-biphenyl]-4-yl]methyl]-L-valine; N-(p-(o-1H-Tetrazol-5-ylphenyl)benzyl)-N-valeryl-L-valine; Tareg; UNII-80M03YXJ7I
|
| Cross-matching ID |
- PubChem CID
- 60846
- PubChem SID
-
7847466
; 11066615
; 11364700
; 11367262
; 11369824
; 11373100
; 11374217
; 11377988
; 11484996
; 11488936
; 11491646
; 11492564
; 11495610
; 14856825
; 14881173
; 26612829
; 26719825
; 43118184
; 46386599
; 46509000
; 46530915
; 47499543
; 48393917
; 49681716
; 49830875
; 50062253
; 50467452
; 53787275
; 57314146
; 81093312
; 85788951
; 90452226
; 92124805
; 92308052
; 92308462
; 92711441
; 93166503
; 99228303
; 103292815
; 103979540
; 104253413
; 104321799
; 117541337
; 117664453
; 119526522
; 123055291
; 124659015
; 124757534
; 124800101
; 125164338
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D06UDG
- Formula
- C24H29N5O3
- Canonical SMILES
- CCCCC(=O)N(CC1=CC=C(C=C1)C2=CC=CC=C2C3=NNN=N3)C(C(C)C)C(=O)O
- InChI
- 1S/C24H29N5O3/c1-4-5-10-21(30)29(22(16(2)3)24(31)32)15-17-11-13-18(14-12-17)19-8-6-7-9-20(19)23-25-27-28-26-23/h6-9,11-14,16,22H,4-5,10,15H2,1-3H3,(H,31,32)(H,25,26,27,28)/t22-/m0/s1
- InChIKey
- ACWBQPMHZXGDFX-QFIPXVFZSA-N
|