| Synonyms |
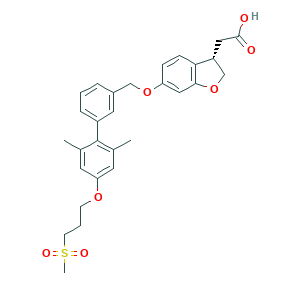
Fasiglifam; GLP1W4JXAH; TAK 875; TAK-875; TAK875; (S)-2-(6-((2',6'-Dimethyl-4'-(3-(methylsulfonyl)propoxy)-[1,1'-biphenyl]-3-yl)methoxy)-2,3-dihydrobenzofuran-3-yl)acetic acid; 1000413-72-8; 3-Benzofuranacetic acid, 6-[[2',6'-diMethyl-4'-[3-(Methylsulfonyl)propoxy][1,1'-biphenyl]-3-yl]Methoxy]-2,3-dihydro-, (3S)-; CHEMBL1829174; UNII-GLP1W4JXAH; [(3s)-6-({2',6'-Dimethyl-4'-[3-(Methylsulfonyl)propoxy]biphenyl-3-Yl}methoxy)-2,3-Dihydro-1-Benzofuran-3-Yl]acetic Acid
|
| Cross-matching ID |
- PubChem CID
- 24857286
- PubChem SID
-
50088191
; 57154181
; 134464228
; 135268194
; 136349531
; 136367662
; 140817135
; 143499812
; 152233038
; 152258229
; 160647065
; 160865873
; 162011413
; 162202766
; 164044689
; 170501170
; 172232430
; 172919610
; 174006778
; 174525708
; 174526154
; 178103098
; 185967135
; 191112353
; 196409677
; 198984389
; 203355926
; 223375341
; 224346112
; 226560798
; 242587051
; 245772988
; 249735423
; 252110185
; 252151428
; 252216368
; 252450486
; 252810271
- CAS Number
-
- TTD Drug ID
- D0V1WQ
- Formula
- C29H32O7S
- Canonical SMILES
- CC1=CC(=CC(=C1C2=CC=CC(=C2)COC3=CC4=C(C=C3)C(CO4)CC(=O)O)C)OCCCS(=O)(=O)C
- InChI
- 1S/C29H32O7S/c1-19-12-25(34-10-5-11-37(3,32)33)13-20(2)29(19)22-7-4-6-21(14-22)17-35-24-8-9-26-23(15-28(30)31)18-36-27(26)16-24/h4,6-9,12-14,16,23H,5,10-11,15,17-18H2,1-3H3,(H,30,31)/t23-/m1/s1
- InChIKey
- BZCALJIHZVNMGJ-HSZRJFAPSA-N
|