| General Information of This Metabolic Reaction (MR) (ID:
MR012897) |
| Formula |
|
| Reactant |
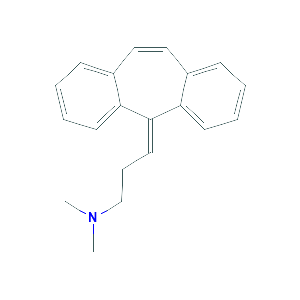
Cyclobenzaprine |
Product |
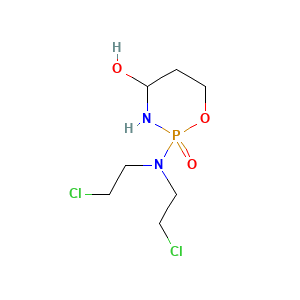
4-Hydroxycyclophosphamide |
|
Reactant Info
|
Product Info
|
|
Metabolic Enzyme
|
Cytochrome P450 2C9 (CYP2C9)
|
DME Info
|
|
Cytochrome P450 3A4 (CYP3A4)
|
DME Info
|
|
Cytochrome P450 2B6 (CYP2B6)
|
DME Info
|
|
Mephenytoin 4-hydroxylase (CYP2C19)
|
DME Info
|
|
Cytochrome P450 3A7 (CYP3A7)
|
DME Info
|
|
Metabolic Type
|
Oxidation
-
4-hydroxylation
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.