| Synonyms |
Aliskiren; Aliskiren fumarate; Aliskiren(CGP 60536); Enviage; Rasilez; Riprazo; SPP 100; SPP100; Sprimeo; Tekturna; (2S,4S,5S,7S)-5-AMINO-N-(2-CARBAMOYL-2-METHYLPROPYL)-4-HYDROXY-2-ISOPROPYL-7-[4-METHOXY-3-(3-METHOXYPROPOXY)BENZYL]-8-METHYLNONANAMIDE; (2S,4S,5S,7S)-5-amino-N-(3-amino-2,2-dimethyl-3-oxopropyl)-4-hydroxy-7-[4-methoxy-3-(3-methoxypropoxy)benzyl]-8-methyl-2-(propan-2-yl)nonanamide; 173334-57-1; 502FWN4Q32; CGP 60536; CGP60536B; CHEBI:601027; CHEMBL1639; UNII-502FWN4Q32
|
| Cross-matching ID |
- PubChem CID
- 5493444
- PubChem SID
-
7980347
; 14763777
; 14886049
; 17397362
; 39475202
; 46507474
; 46509572
; 51009124
; 85176969
; 87324641
; 96099933
; 103615727
; 104178818
; 114001043
; 124897540
; 126666078
; 129435102
; 134338459
; 135916045
; 137002989
; 137227121
; 140385193
; 151991991
; 152028160
; 152258402
; 160647239
; 160964589
; 162181387
; 174006809
; 175268617
; 176485078
; 177748803
; 178101514
; 179150066
; 185997039
; 187032771
; 223393380
; 223404348
; 223435288
; 223661693
; 223720068
; 224032552
; 226775246
; 242591596
; 251916704
; 251917943
; 251970968
; 252213891
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D03SVX
- Formula
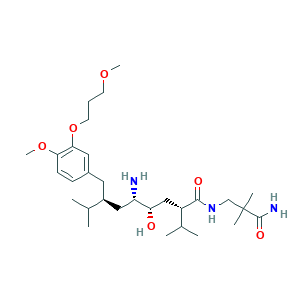
- C30H53N3O6
- Canonical SMILES
- CC(C)C(CC1=CC(=C(C=C1)OC)OCCCOC)CC(C(CC(C(C)C)C(=O)NCC(C)(C)C(=O)N)O)N
- InChI
- 1S/C30H53N3O6/c1-19(2)22(14-21-10-11-26(38-8)27(15-21)39-13-9-12-37-7)16-24(31)25(34)17-23(20(3)4)28(35)33-18-30(5,6)29(32)36/h10-11,15,19-20,22-25,34H,9,12-14,16-18,31H2,1-8H3,(H2,32,36)(H,33,35)/t22-,23-,24-,25-/m0/s1
- InChIKey
- UXOWGYHJODZGMF-QORCZRPOSA-N
|