| Synonyms |
Anastrozol; Anastrozole, 98%; Arimidex; YBBLVLTVTVSKRW-UHFFFAOYSA-N; ZD 1033; ZD-1033; ZD1033; anastrazole; anastrozole; 120511-73-1; 2,2'-(5-((1H-1,2,4-triazol-1-yl)methyl)-1,3-phenylene)bis(2-methylpropanenitrile); 2-[3-(2-cyanopropan-2-yl)-5-(1,2,4-triazol-1-ylmethyl)phenyl]-2-methylpropanenitrile; 2Z07MYW1AZ; C17H19N5; CHEBI:2704; CHEMBL1399; HSDB 7462; ICI D1033; ICI-D 1033; ICI-D1033; NSC719344; UNII-2Z07MYW1AZ; alpha,alpha,alpha',alpha'-Tetramethyl-5-(1H-1,2,4-triazol-1-ylmethyl)-m-benzenediacetonitrile
|
| Cross-matching ID |
- PubChem CID
- 2187
- PubChem SID
-
10359
; 535026
; 5128740
; 7848023
; 7978492
; 8151486
; 11528617
; 12014321
; 14751420
; 26719808
; 26758041
; 29221365
; 46386543
; 46504987
; 46510669
; 48415567
; 49681575
; 53007586
; 53789173
; 56311273
; 56313558
; 56313576
; 57321190
; 58106884
; 81040864
; 87351875
; 92308653
; 92308946
; 92712142
; 99436928
; 103478231
; 104299862
; 117672710
; 118313740
; 124659088
; 124757065
; 124801312
; 125163869
; 125311691
; 125339200
; 126630179
; 126657740
; 126667645
; 129769972
; 131296284
; 134337917
; 135018130
; 135698190
; 135727309
; 136348802
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0W0BF
- Formula
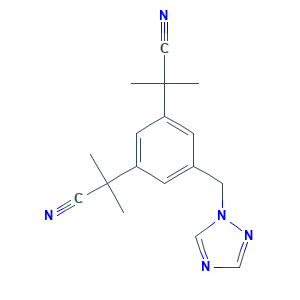
- C17H19N5
- Canonical SMILES
- CC(C)(C#N)C1=CC(=CC(=C1)CN2C=NC=N2)C(C)(C)C#N
- InChI
- 1S/C17H19N5/c1-16(2,9-18)14-5-13(8-22-12-20-11-21-22)6-15(7-14)17(3,4)10-19/h5-7,11-12H,8H2,1-4H3
- InChIKey
- YBBLVLTVTVSKRW-UHFFFAOYSA-N
|