| Synonyms |
Asunaprevir; Asunaprevir (BMS-650032); Asunaprevir [USAN:INN]; S9X0KRJ00S; Sunvepra (TN); 2R9; 630420-16-5; BMS 650032; BMS-650032; N-(Tert-Butoxycarbonyl)-3-Methyl-L-Valyl-(4r)-4-[(7-Chloro-4-Methoxyisoquinolin-1-Yl)oxy]-N-{(1r,2s)-1-[(Cyclopropylsulfonyl)carbamoyl]-2-Ethenylcyclopropyl}-L-Prolinamide; UNII-S9X0KRJ00S; tert-butyl N-[(1S)-1-[(2S,4R)-4-[(7-chloro-4-methoxy-1-isoquinolyl)oxy]-2-[[(1R,2S)-1-(cyclopropylsulfonylcarbamoyl)-2-vinyl-cyclopropyl]carbamoyl]pyrrolidine-1-carbonyl]-2,2-dimethyl-propyl]carbamate
|
| Cross-matching ID |
- PubChem CID
- 16076883
- PubChem SID
-
24721638
; 28688599
; 29960656
; 81082068
; 99431640
; 135316582
; 135626812
; 139010040
; 152258574
; 160647409
; 160692698
; 174315703
; 198943682
; 198993976
; 210024640
; 210024643
; 223435540
; 223715301
; 224325540
; 228723059
; 242681705
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D04MCY
- Formula
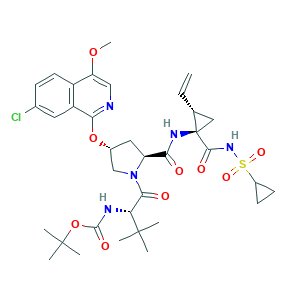
- C35H46ClN5O9S
- Canonical SMILES
- CC(C)(C)C(C(=O)N1CC(CC1C(=O)NC2(CC2C=C)C(=O)NS(=O)(=O)C3CC3)OC4=NC=C(C5=C4C=C(C=C5)Cl)OC)NC(=O)OC(C)(C)C
- InChI
- 1S/C35H46ClN5O9S/c1-9-19-16-35(19,31(44)40-51(46,47)22-11-12-22)39-28(42)25-15-21(49-29-24-14-20(36)10-13-23(24)26(48-8)17-37-29)18-41(25)30(43)27(33(2,3)4)38-32(45)50-34(5,6)7/h9-10,13-14,17,19,21-22,25,27H,1,11-12,15-16,18H2,2-8H3,(H,38,45)(H,39,42)(H,40,44)/t19-,21-,25+,27-,35-/m1/s1
- InChIKey
- XRWSZZJLZRKHHD-WVWIJVSJSA-N
|