| General Information of Drug (ID:
DR0753) |
| Drug Name |
Fospropofol
|
| Synonyms |
Fospropofol; Fospropofol [INN]; LZ257RZP7K; SCHEMBL3821874; ZINC2519740; 258516-89-1; AC1MI4YI; ACM258516891; CHEBI:135193; CHEMBL1201766; DB06716; DEA No. 2138; DTXSID50870295; FT-0727559; GTPL7475; UNII-LZ257RZP7K; [2,6-di(propan-2-yl)phenoxy]methyl dihydrogen phosphate
|
| Indication |
Neuropathic pain
[ICD11: 8E43]
|
Approved
|
[1]
|
| Structure |
|
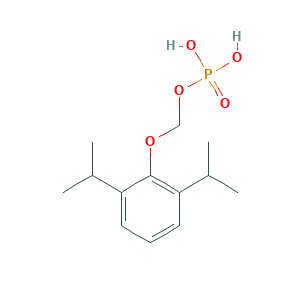 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
288.28 |
Topological Polar Surface Area |
76 |
| Heavy Atom Count |
19 |
Rotatable Bond Count |
6 |
| Hydrogen Bond Donor Count |
2 |
Hydrogen Bond Acceptor Count |
5 |
| Cross-matching ID |
- PubChem CID
- 3038498
- ChEBI ID
-
- CAS Number
-
- Formula
- C13H21O5P
- Canonical SMILES
- CC(C)C1=C(C(=CC=C1)C(C)C)OCOP(=O)(O)O
- InChI
- 1S/C13H21O5P/c1-9(2)11-6-5-7-12(10(3)4)13(11)17-8-18-19(14,15)16/h5-7,9-10H,8H2,1-4H3,(H2,14,15,16)
- InChIKey
- QVNNONOFASOXQV-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


