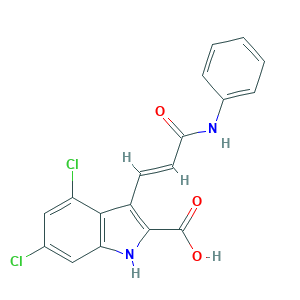
| Synonyms |
Gavestinel; Gavestinel (USAN/INN); Gavestinel [USAN:INN:BAN]; SCHEMBL1721070; SCHEMBL678632; (E)-4,6-Dichloro-3-(3-oxo-3-(phenylamino)-1-propenyl)-1H-indole-2-carboxylic acid; 153436-22-7; 1H-Indole-2-carboxylic acid, 4,6-dichloro-3-((1E)-3-oxo-3-(phenylamino)-1-propenyl)-; 1H-Indole-2-carboxylic acid, 4,6-dichloro-3-(3-oxo-3-(phenylamino)-1-propenyl)-, (E)-; 318X4QY113; 4,6-Dichloro-3-((E)-2-(phenylcarbamoyl)vinyl)indole-2-carboxylic acid; AC1O51WR; CHEMBL44793; GV 150526; GV 150526X; GV-150526X; UNII-318X4QY113
|
| Cross-matching ID |
- PubChem CID
- 6450546
- PubChem SID
-
11972111
; 14877626
; 43041642
; 47206237
; 49983607
; 50438457
; 57370215
; 85788700
; 103227166
; 103956928
; 114525294
; 134339038
; 134340043
; 135236023
; 137132921
; 137249725
; 142472724
; 162221852
; 179322268
; 184596079
; 198982944
; 226973454
; 227903014
; 242057623
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0O6AQ
- Formula
- C18H12Cl2N2O3
- Canonical SMILES
- C1=CC=C(C=C1)NC(=O)C=CC2=C(NC3=CC(=CC(=C32)Cl)Cl)C(=O)O
- InChI
- 1S/C18H12Cl2N2O3/c19-10-8-13(20)16-12(17(18(24)25)22-14(16)9-10)6-7-15(23)21-11-4-2-1-3-5-11/h1-9,22H,(H,21,23)(H,24,25)/b7-6+
- InChIKey
- WZBNEZWCNKUOSM-VOTSOKGWSA-N
|