| Cross-matching ID |
- PubChem CID
- 3488
- PubChem SID
-
9234
; 855894
; 3146598
; 5047958
; 7847402
; 7979403
; 8147024
; 8150168
; 8152216
; 10321542
; 10502519
; 11111219
; 11112597
; 11113353
; 11119957
; 11120445
; 11120933
; 11121416
; 11121896
; 11147040
; 11335656
; 11360895
; 11362485
; 11363144
; 11365047
; 11365706
; 11367609
; 11368268
; 11370241
; 11370242
; 11372372
; 11373210
; 11375035
; 11375771
; 11376430
; 11461867
; 11466344
; 11467464
; 11485567
; 11486267
; 11489632
; 11491142
; 11493013
; 11494064
; 14835352
; 17405062
; 22391414
; 24277838
; 24895097
; 26542333
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D05LYX
- Formula
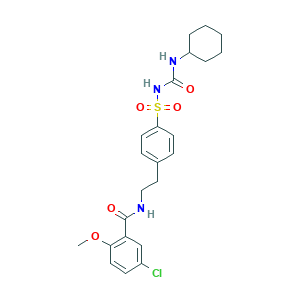
- C23H28ClN3O5S
- Canonical SMILES
- COC1=C(C=C(C=C1)Cl)C(=O)NCCC2=CC=C(C=C2)S(=O)(=O)NC(=O)NC3CCCCC3
- InChI
- 1S/C23H28ClN3O5S/c1-32-21-12-9-17(24)15-20(21)22(28)25-14-13-16-7-10-19(11-8-16)33(30,31)27-23(29)26-18-5-3-2-4-6-18/h7-12,15,18H,2-6,13-14H2,1H3,(H,25,28)(H2,26,27,29)
- InChIKey
- ZNNLBTZKUZBEKO-UHFFFAOYSA-N
|