| General Information of Drug (ID:
DR0776) |
| Drug Name |
Gliclazide
|
| Synonyms |
Gliclazida; Gliclazida [INN-Spanish]; Gliclazidum; Gliclazidum [INN-Latin]; Glimicron; Diamicron; Nordialex; gliclazide; 1-(3-Azabicyclo(3.3.0)oct-3-yl)-3-(p-tolylsulfonyl)urea; 1-(3-Azabicyclo[3.3.0]oct-3-yl)-3-p-tolylsulphonylurea; 1-(Hexahydrocyclopenta(c)pyrrol-2(1H)-yl)-3-(p-tolylsulfonyl)urea; 21187-98-4; C15H21N3O3S; CHEBI:31654; MFCD00409893; N-(4-Methylbenzenesulfonyl)-N'-(3-azabicyclo(3.3.0)oct-3-yl)urea; N-(hexahydrocyclopenta[c]pyrrol-2(1H)-ylcarbamoyl)-4-methylbenzenesulfonamide
|
| Indication |
Diabetes mellitus
[ICD11: 5A10]
|
Phase 4
|
[1]
|
| Structure |
|
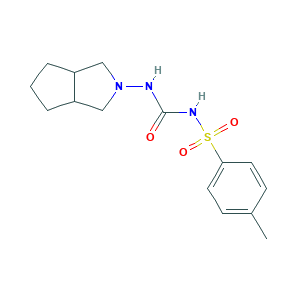 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
323.4 |
Topological Polar Surface Area |
86.9 |
| Heavy Atom Count |
22 |
Rotatable Bond Count |
3 |
| Hydrogen Bond Donor Count |
2 |
Hydrogen Bond Acceptor Count |
4 |
| Cross-matching ID |
- PubChem CID
- 3475
- PubChem SID
-
3206372
; 5035155
; 7848662
; 7979407
; 8152209
; 10321809
; 11342141
; 11362324
; 11364372
; 11366934
; 11369496
; 11372903
; 11374235
; 11377658
; 11466586
; 11467706
; 11486292
; 11487726
; 11491677
; 11492413
; 11495292
; 11533945
; 14826273
; 24895090
; 26612635
; 26612723
; 26748918
; 26748919
; 26748920
; 29222608
; 47656599
; 47730748
; 48179242
; 48179243
; 48328563
; 48403959
; 48416054
; 49646130
; 49698833
; 50061218
; 50086570
; 50107481
; 50107482
; 50107483
; 50880518
; 57321824
; 81040947
; 81066070
; 81093129
; 85280584
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0M2MR
- Formula
- C15H21N3O3S
- Canonical SMILES
- CC1=CC=C(C=C1)S(=O)(=O)NC(=O)NN2CC3CCCC3C2
- InChI
- 1S/C15H21N3O3S/c1-11-5-7-14(8-6-11)22(20,21)17-15(19)16-18-9-12-3-2-4-13(12)10-18/h5-8,12-13H,2-4,9-10H2,1H3,(H2,16,17,19)
- InChIKey
- BOVGTQGAOIONJV-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


