| Synonyms |
Pentamidine (isethionate); Pentamidine diisethionate; Pentamidine isethionate salt; Pentamidine isothionate; Pentaminide isetionate; USAF XR-10; V2P3K60DA2; 1,5-Bis(p-amidinophenoxy)pentane bis(2-hydroxyethanesulfonate salt); 140-64-7; 4,4'-(Pentamethylenedioxy)dibenzamidine bis(2-hydroxyethanesulfonate); 4,4'-(Pentane-1,5-diylbis(oxy))dibenzimidamide bis(2-hydroxyethanesulfonate); CCRIS 1660; CHEBI:7977; MFCD00079213; UNII-V2P3K60DA2; PENTAMIDINE ISETHIONATE; Nebupent (as isethionate); Pentacarinat (as isethionate); Pentam 300 (as isethionate); Pentamide; Pentamidin; Pentamidina; Pentamidina [DCIT]; Pentamidine [INN:BAN:DCF]; Pentamidine mesylate; Pentamidinum; Pentamidinum [INN-Latin]; pentamidine; 100-33-4; 4,4'-(Pentamethylenedioxy)dibenzamidine; 4,4'-(Pentane-1,5-diylbis(oxy))dibenzimidamide; 4,4'-Diamidinodiphenoxypentane; CCRIS 3825; UNII-673LC5J4LQ; p,p'-(Pentamethylenedioxy)dibenzamidine
|
| Cross-matching ID |
- PubChem CID
- 8813
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D05EAM
- Formula
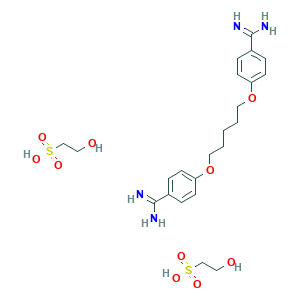
- C23H36N4O10S2
- Canonical SMILES
- C1=CC(=CC=C1C(=N)N)OCCCCCOC2=CC=C(C=C2)C(=N)N.C(CS(=O)(=O)O)O.C(CS(=O)(=O)O)O
- InChI
- 1S/C19H24N4O2.2C2H6O4S/c20-18(21)14-4-8-16(9-5-14)24-12-2-1-3-13-25-17-10-6-15(7-11-17)19(22)23;2*3-1-2-7(4,5)6/h4-11H,1-3,12-13H2,(H3,20,21)(H3,22,23);2*3H,1-2H2,(H,4,5,6)
- InChIKey
- YBVNFKZSMZGRAD-UHFFFAOYSA-N
|