| General Information of Drug (ID:
DR1393) |
| Drug Name |
Ranitidine
|
| Synonyms |
Radinat; Randin; Ranidine; Raniogas; Ranisen; Raniter; Ranitidin; Ranitidina; Ranitidina [INN-Spanish]; Ranitidine Base; Ranitidine HCL; Ranitidinum; Ranitidinum [INN-Latin]; Ranitiget; Rantacid; Rantidine; Raticina; Sampep; Sostril; Achedos; Acidex; Atural; Axoban; Coralen; Duractin; Ezopta; Gastrial; Gastrosedol; Istomar; Logast; Mauran; Microtid; Ptinolin; Quantor; Quicran; Taural; Ul-Pep; Ulceranin; Urantac; Verlost; Vesyca; Vizerul; Weichilin; Weidos; Xanidine; ZANTAC; Zantab; Zantadin; Zantic; ranitidine; 66357-35-5; Ranin; Ratic
|
| Indication |
Peptic ulcer
[ICD11: DA61]
|
Approved
|
[1]
|
| Structure |
|
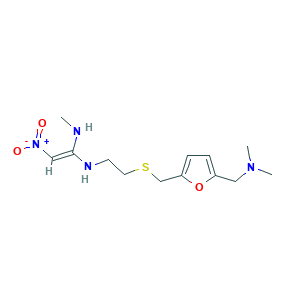 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
314.41 |
Topological Polar Surface Area |
112 |
| Heavy Atom Count |
21 |
Rotatable Bond Count |
9 |
| Hydrogen Bond Donor Count |
2 |
Hydrogen Bond Acceptor Count |
7 |
| Cross-matching ID |
- PubChem CID
- 3001055
- PubChem SID
-
615112
; 7847488
; 7980484
; 8149747
; 10036397
; 10532147
; 11111714
; 11111715
; 11113371
; 14776753
; 26612202
; 26680173
; 26748519
; 26748520
; 26753732
; 34666962
; 46505543
; 47216723
; 47515261
; 47810695
; 48184944
; 49846707
; 49968693
; 50100822
; 50107423
; 50107424
; 50139267
; 50286426
; 53790194
; 57410138
; 85209253
; 85245763
; 90341455
; 92124471
; 92307665
; 92711914
; 93166337
; 99301528
; 103091649
; 103155999
; 103173566
; 104171226
; 111610678
; 117377112
; 117814891
; 118258804
; 124551883
; 124636867
; 124881290
; 124881291
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0B8WN
- Formula
- C13H22N4O3S
- Canonical SMILES
- CNC(=C[N+](=O)[O-])NCCSCC1=CC=C(O1)CN(C)C
- InChI
- 1S/C13H22N4O3S/c1-14-13(9-17(18)19)15-6-7-21-10-12-5-4-11(20-12)8-16(2)3/h4-5,9,14-15H,6-8,10H2,1-3H3/b13-9+
- InChIKey
- VMXUWOKSQNHOCA-UKTHLTGXSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


