| Synonyms |
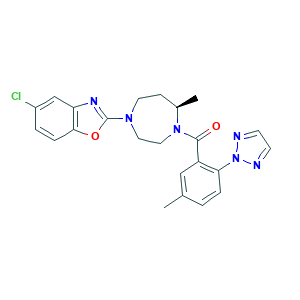
Suvorexant; Suvorexant (MK-4305); (R)-(4-(5-Chlorobenzo[d]oxazol-2-yl)-7-methyl-1,4-diazepan-1-yl)(5-methyl-2-(2H-1,2,3-triazol-2-yl)phenyl)methanone; BELSOMRA; MK 4305; MK-4305; MK4305; 081L192FO9; 1030377-33-3; 5-Chloro-2-[(5R)-5-methyl-4-[5-methyl-2-(2H-1,2,3-triazol-2-yl)benzoyl]-1,4-diazepan-1-yl]-1,3-benzoxazole; C23H23ClN6O2; CHEBI:82698; CHEMBL1083659; UNII-081L192FO9; [(7R)-4-(5-Chloro-1,3-benzoxazol-2-yl)-7-methyl-1,4-diazepan-1-yl][5-methyl-2-(2H-1,2,3-triazol-2-yl)phenyl]methanone
|
| Cross-matching ID |
- PubChem CID
- 24965990
- PubChem SID
-
56264110
; 57493879
; 99431637
; 103755866
; 104153062
; 134348113
; 135267387
; 135650615
; 136946578
; 138669889
; 141618580
; 152258025
; 152258268
; 160647104
; 163312227
; 164044652
; 171061162
; 175266695
; 176245483
; 184816450
; 198993974
; 215784570
; 223377940
; 223447420
; 223620167
; 223704064
; 225372552
; 227781057
; 248624194
; 249822123
; 250214353
; 251963163
; 252062692
; 252211821
; 252215820
; 252442778
; 252473529
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D00OVU
- Formula
- C23H23ClN6O2
- Canonical SMILES
- CC1CCN(CCN1C(=O)C2=C(C=CC(=C2)C)N3N=CC=N3)C4=NC5=C(O4)C=CC(=C5)Cl
- InChI
- 1S/C23H23ClN6O2/c1-15-3-5-20(30-25-8-9-26-30)18(13-15)22(31)29-12-11-28(10-7-16(29)2)23-27-19-14-17(24)4-6-21(19)32-23/h3-6,8-9,13-14,16H,7,10-12H2,1-2H3/t16-/m1/s1
- InChIKey
- JYTNQNCOQXFQPK-MRXNPFEDSA-N
|