| General Information of Drug (ID:
DR1710) |
| Drug Name |
Vorinostat
|
| Synonyms |
Vorinostat; Vorinostat (SAHA); Vorinostat (SAHA, MK0683); WAEXFXRVDQXREF-UHFFFAOYSA-N; WIN64652; Zolinza; suberoylanilide hydroxamic acid; MK-0683; MK0683; N'-hydroxy-N-phenyloctanediamide; N-Hyrdroxy-N'-phenyloctanediamide; N-hydroxy-N'-phenyloctanediamide; N1-hydroxy-N8-phenyloctanediamide; SAHA cpd; Suberanilohydroxamic acid; 149647-78-9; 58IFB293JI; CCRIS 8456; CHEBI:45716; CHEMBL98; MFCD00945317; NSC-701852; NSC701852; OCTANEDIOIC ACID HYDROXYAMIDE PHENYLAMIDE; Octanediamide, N-hydroxy-N'-phenyl-; SAHA; SHH; UNII-58IFB293JI
|
| Indication |
Cutaneous T-cell lymphoma
[ICD11: 2B01]
|
Approved
|
[1]
|
| Structure |
|
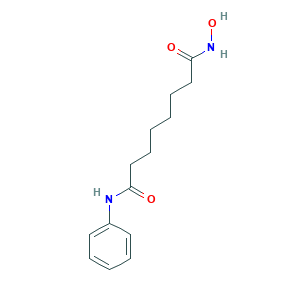 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
264.32 |
Topological Polar Surface Area |
78.4 |
| Heavy Atom Count |
19 |
Rotatable Bond Count |
8 |
| Hydrogen Bond Donor Count |
3 |
Hydrogen Bond Acceptor Count |
3 |
| Cross-matching ID |
- PubChem CID
- 5311
- PubChem SID
-
830547
; 832844
; 5620782
; 7890463
; 8153256
; 12015019
; 14710657
; 14823867
; 26684150
; 29224365
; 46393757
; 46508989
; 46519184
; 47207977
; 48426290
; 49645508
; 50070170
; 50070537
; 50071316
; 50113029
; 53788318
; 56311712
; 56312417
; 56312644
; 56314569
; 57322702
; 68530763
; 75748492
; 85736432
; 87226482
; 92708291
; 92719823
; 99350949
; 99436957
; 103174146
; 104170365
; 104171411
; 104308873
; 118048589
; 121362164
; 124756962
; 124766849
; 124795966
; 124893441
; 124893442
; 125163768
; 125335517
; 126591282
; 126628079
; 126649066
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0E7PQ
- Formula
- C14H20N2O3
- Canonical SMILES
- C1=CC=C(C=C1)NC(=O)CCCCCCC(=O)NO
- InChI
- 1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18)
- InChIKey
- WAEXFXRVDQXREF-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


