Details of the Drug Metabolized by Drug-Metabolizing Enzyme (DME)
| General Information of Drug (ID: DR1869) | ||||||
|---|---|---|---|---|---|---|
| Drug Name |
ARQ-501
|
|||||
| Synonyms |
ARQ 501; ARQ-501; Lapachone, beta-; SL 11001; beta-Lapachone; .beta.-Lapachone; 2,2-Dimethyl-3,4-dihydro-2H-benzo[h]chromene-5,6-dione; 2,2-dimethyl-3,4-dihydrobenzo[h]chromene-5,6-dione; 2H-Naphtho[1,2-b]pyran-5,6-dione, 3,4-dihydro-2,2-dimethyl-; 3,4-Dihydro-2,2-dimethyl-2H-naphtho(1,2-b)pyran-5,6-dione; 3,4-Dihydro-2,2-dimethyl-2H-naphtho[1,2-B]pyran-5,6-dione; 3,4-Dihydro-2,2-dimethyl-2H-naphthol[1,2-b]pyran-5,6-dione; 4707-32-8; 6N4FA2QQ6A; BRN 0181499; CHEBI:10429; NSC 26326; NSC 629749; UNII-6N4FA2QQ6A
|
|||||
| Indication | Leiomyosarcoma [ICD11: 2B58] | Phase 2 | [1] | |||
| Structure |
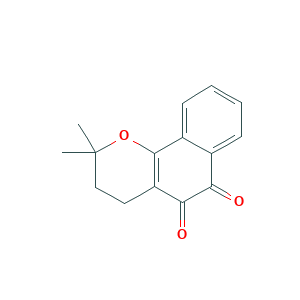 |
|||||
| 3D MOL | 2D MOL | |||||
| Pharmaceutical Properties | Molecular Weight | 242.27 | Topological Polar Surface Area | 43.4 | ||
| Heavy Atom Count | 18 | Rotatable Bond Count | 0 | |||
| Hydrogen Bond Donor Count | 0 | Hydrogen Bond Acceptor Count | 3 | |||
| Cross-matching ID |
|
|||||
| The Metabolic Roadmap of This Drug | |||||
|---|---|---|---|---|---|
| The Full List of Drug Metabolites (DM) of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| The Full List of Metabolic Reaction (MR) of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug-Metabolizing Enzyme(s) (DME) Metabolizing This Drug | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.

