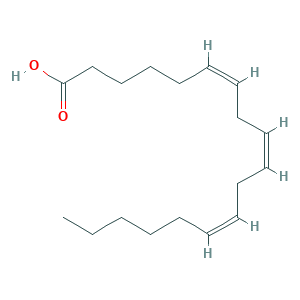
| Synonyms |
Acide gamolenique [French]; Acido gamolenico [Spanish]; Acidum gamolenicum [Latin]; GAMOLENIC ACID; Gamolenic acid [INN:BAN]; gamma-Linolenic acid; (6,9,12)-linolenic acid; (6Z,9Z,12Z)-octadeca-6,9,12-trienoic acid; (Z,Z,Z)-6,9,12-Octadecatrienoic acid; 506-26-3; 6,9,12-Octadecatrienoic acid, (Z,Z,Z)-; 6Z,9Z,12Z-octadecatrienoic acid; 78YC2MAX4O; CCRIS 7668; CHEBI:28661; CHEMBL464982; Ligla; UNII-78YC2MAX4O; all-cis-6,9,12-Octadecatrienoic acid; cis,cis,cis-6,9,12-Octadecatrienoic acid; cis-Delta(6,9,12)-octadecatrienoic acid
|
| Cross-matching ID |
- PubChem CID
- 5280933
- PubChem SID
-
8661
; 615077
; 841809
; 7850008
; 7979368
; 8144304
; 8616448
; 12014288
; 14775234
; 24882200
; 24896312
; 26754623
; 26754624
; 29216151
; 39289902
; 47290899
; 47885167
; 49998727
; 50110102
; 51068121
; 51091551
; 53787075
; 57260207
; 57264360
; 57357934
; 85787690
; 87572103
; 91700727
; 92298626
; 92309754
; 99300668
; 99302186
; 103636892
; 104046527
; 104115203
; 113854242
; 119525823
; 124799300
; 126524717
; 134976478
; 137006167
; 141706287
; 144205388
; 162180888
; 163781850
; 164223441
; 164816041
; 175266274
; 178101421
; 179117054
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0UE9X
- Formula
- C18H30O2
- Canonical SMILES
- CCCCCC=CCC=CCC=CCCCCC(=O)O
- InChI
- 1S/C18H30O2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18(19)20/h6-7,9-10,12-13H,2-5,8,11,14-17H2,1H3,(H,19,20)/b7-6-,10-9-,13-12-
- InChIKey
- VZCCETWTMQHEPK-QNEBEIHSSA-N
|