| Synonyms |
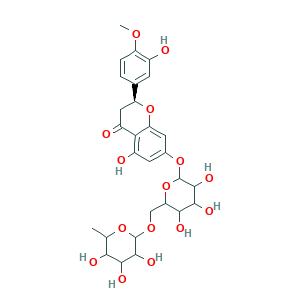
Hesperetin 7-O-rutinoside; LMPK12140451; MCULE-6401179754; QUQPHWDTPGMPEX-SGHBVPQBSA-N; STK801624; Hesperetin-7-O-rutinoside; hesperidin; (2S)-5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-4-oxo-3,4-dihydro-2H-chromen-7-yl 6-O-(6-deoxyhexopyranosyl)hexopyranoside; 520-26-3; AB0013409; AKOS004119862; AKOS016357494; Flavanone, 3',5,7-trihydroxy-4'-methoxy-, 7-(6-O-.alpha.-L-rhamnosyl-D-glucoside); H6243; H992; ALBB-028539; BBL033922; SCHEMBL13886768
|
| Cross-matching ID |
- PubChem CID
- 16394497
- CAS Number
-
- Formula
- C28H34O15
- Canonical SMILES
- CC1C(C(C(C(O1)OCC2C(C(C(C(O2)OC3=CC(=C4C(=O)CC(OC4=C3)C5=CC(=C(C=C5)OC)O)O)O)O)O)O)O)O
- InChI
- 1S/C28H34O15/c1-10-21(32)23(34)25(36)27(40-10)39-9-19-22(33)24(35)26(37)28(43-19)41-12-6-14(30)20-15(31)8-17(42-18(20)7-12)11-3-4-16(38-2)13(29)5-11/h3-7,10,17,19,21-30,32-37H,8-9H2,1-2H3/t10?,17-,19?,21?,22?,23?,24?,25?,26?,27?,28?/m0/s1
- InChIKey
- QUQPHWDTPGMPEX-SGHBVPQBSA-N
|