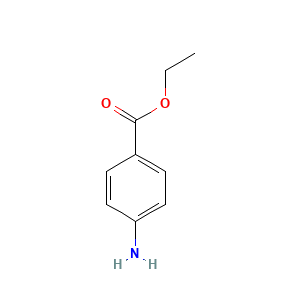
| Synonyms |
Aethoform; Americaine; Anaesthesin; Anaesthesinum; Anaesthin; Anestezin; Anesthesin; Anesthesine; Anesthone; Bensokain; Benzocaina; Benzocainum; Chloraseptic; Dermoplast; Ethoform; Ethoforme; Hurricaine; Identhesin; Keloform; Norcain; Norcaine; Norcainum; Orthesin; Otocain; Outgro; Parathesin; Parathesine; Solarcaine; Topcaine; Aethylium paraminobenzoicum; Amben ethyl ester; Anestezin [Russian]; Baby Anbesol; Benzocaine Acetate; Benzocaine Formate; Benzocaine Hydrobromide; Benzocaine Hydrochloride; Benzocaine Methanesulfonate; Ethyl PABA; Ethyl aminobenzoate; Ethylis aminobenzoas; Solu H; Acetate, Benzocaine; Anaesthan-syngala; Auralgan (TN); Benzocaina [INN-Spanish]; Benzocaine [INN:BAN]; Benzocainum [INN-Latin]; Ethyl 4-aminobenzoate; Ethyl 4-aminobenzoate hydrochloride; Ethyl aminobenzoate (JP15); Ethyl aminobenzoate (VAN); Ethyl p-aminobenzoate; Ethyl p-aminophenylcarboxylate; Ethylaminobenzoate-4; Ethylesterkyseliny p-aminobenzoove; Ethylester kyseliny p-aminobenzoove [Czech]; Formate, Benzocaine; Hydrobromide, Benzocaine; Hydrochloride, Benzocaine; Methanesulfonate, Benzocaine; Ora-jel; P-Aminobenzoic acid ethyl ester; P-Aminobenzoic ethyl ester; P-Carbethoxyaniline; P-Ethoxycarboxylic aniline; Parathesin (TN); AE-562/40377256; Benzocaine (USP/INN); ETHYL-P-AMINOBENZOATE; P-(Ethoxycarbonyl)aniline;P-Aminobenzoic acid, ethyl ester; Benzoic acid, amino-, ethyl ester; H-4-abz-oet; Benzoic acid, 4-amino-, ethyl ester; Benzoic acid, p-amino-, ethyl ester; Benzoic acid, 4-amino-, ethyl ester, hydrochloride; 112909_ALDRICH; 112909_SIAL; 4 Aminobenzoic Acid Ethyl Ester; 4-(Ethoxycarbonyl)aniline; 4-Aminobenzoic acid ethyl ester; 4-Aminobenzoic acid, ethyl ester; 4-Carbethoxyaniline; 4-amino-benzoic acid ethyl ester
|