| General Information of Drug (ID:
DR0027) |
| Drug Name |
Abacavir
|
| Synonyms |
Abacavir (INN); Abacavir [INN:BAN]; Abacavir sulfate; CHEBI:421707; MFCD00903850; NSC742406; UNII-WR2TIP26VS; [(1S,4R)-4-[2-amino-6-(cyclopropylamino)purin-9-yl]cyclopent-2-en-1-yl]methanol; abacavir; {(1S-cis)-4-[2-amino-6-(cyclopropylamino)-9H-purin-9-yl]cyclopent-2-en-1-yl}methanol; Ziagen; (+/-)-Abacavir; (1S,4R)-4-[2-Amino-6-(cyclopropylamino)-9H-purin-9-yl]-2-cyclopentene-1-methanol; 136470-78-5; 1592U89; 2-Cyclopentene-1-methanol, 4-[2-amino-6-(cyclopropylamino)-9H-purin-9-yl]-, (1S,4R)-; WR2TIP26VS
|
| Indication |
Human immunodeficiency virus infection
[ICD11: 1C60]
|
Approved
|
[1]
|
| Structure |
|
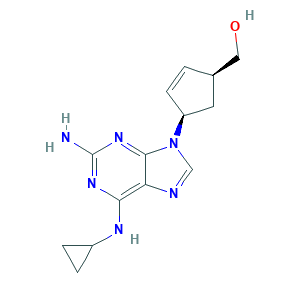 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
286.33 |
Topological Polar Surface Area |
102 |
| Heavy Atom Count |
21 |
Rotatable Bond Count |
4 |
| Hydrogen Bond Donor Count |
3 |
Hydrogen Bond Acceptor Count |
6 |
| Cross-matching ID |
- PubChem CID
- 441300
- PubChem SID
-
9826
; 603460
; 612310
; 7978623
; 10298786
; 14775540
; 14799909
; 26757979
; 26757980
; 36885172
; 46505718
; 51091399
; 57403623
; 70504220
; 76364492
; 81092778
; 85612588
; 92729708
; 103463308
; 104052305
; 104625144
; 126654052
; 128575040
; 131298145
; 135025044
; 136345668
; 137006087
; 142178555
; 160845918
; 160964383
; 162011475
; 162176802
; 163621096
; 163686421
; 163883088
; 164814948
; 165235811
; 175267614
; 175442679
; 179149865
; 184545024
; 186014779
; 196109919
; 223516662
; 223703089
; 223704757
; 223900945
; 226424139
; 241033176
; 241383802
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0A4IJ
- Formula
- C14H18N6O
- Canonical SMILES
- C1CC1NC2=NC(=NC3=C2N=CN3C4CC(C=C4)CO)N
- InChI
- 1S/C14H18N6O/c15-14-18-12(17-9-2-3-9)11-13(19-14)20(7-16-11)10-4-1-8(5-10)6-21/h1,4,7-10,21H,2-3,5-6H2,(H3,15,17,18,19)/t8-,10+/m1/s1
- InChIKey
- MCGSCOLBFJQGHM-SCZZXKLOSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


