Details of the Drug Metabolized by Drug-Metabolizing Enzyme (DME)
| General Information of Drug (ID: DR0554) | ||||||
|---|---|---|---|---|---|---|
| Drug Name |
Duloxetine hydrochloride
|
|||||
| Synonyms |
Duloxetine (hydrochloride); Duloxetine HCl; Duloxetine hydrochloride [USAN]; Duloxetine; (3S)-N-methyl-3-(1-naphthyloxy)-3-(2-thienyl)propan-1-amine; (S)-Duloxetine; (S)-N-Methyl-3-(naphthalen-1-yloxy)-3-(thiophen-2-yl)propan-1-amine; (S)-N-Methyl-gamma-(1-naphthalenyloxy)-2-thiophenepropanamine; 116539-59-4; CHEBI:36795; CPD000449282; Duloxetine [INN:BAN]; HSDB 7368; LY 248686; LY248686; MFCD06801358; N-Methyl-gama-(1-naphthalenyloxy)-2-thiophenepropanamine; O5TNM5N07U; UNII-O5TNM5N07U; Yentreve; LY-248686; Q-102508; UNII-9044SC542W; (3S)-N-methyl-3-(1-naphthyloxy)-3-(2-thienyl)propan-1-amine hydrochloride; (S)-Duloxetine HCl; (S)-N-Methyl-3-(naphthalen-1-yloxy)-3-(thiophen-2-yl)propan-1-amine hydrochloride; (S)-duloxetine hydrochloride; 136434-34-9; 9044SC542W; C18H19NOS.HCl; C18H20ClNOS; CHEBI:31526; CPD000469136; DSSTox_CID_26443; DSSTox_GSID_46443; DSSTox_RID_81618
|
|||||
| Indication | Depression [ICD11: 6A71] | Approved | [1] | |||
| Structure |
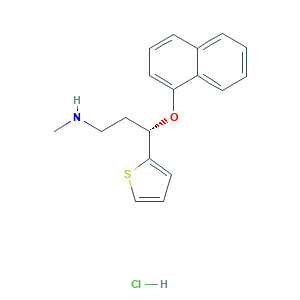 |
|||||
| 3D MOL is unavailable | 2D MOL | |||||
| Pharmaceutical Properties | Molecular Weight | 333.9 | Topological Polar Surface Area | 49.5 | ||
| Heavy Atom Count | 22 | Rotatable Bond Count | 6 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 3 | |||
| Cross-matching ID |
|
|||||
| The Metabolic Roadmap of This Drug | |||||
|---|---|---|---|---|---|
| The Full List of Drug Metabolites (DM) of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug-Metabolizing Enzyme(s) (DME) Metabolizing This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | ||||||
|---|---|---|---|---|---|---|
| 1 | Duloxetine Hydrochloride was approved by FDA. The 2020 official website of the U.S. Food and Drug Administration. | |||||
| 2 | Duloxetine: clinical pharmacokinetics and drug interactions. Clin Pharmacokinet. 2011 May;50(5):281-94. | |||||
| 3 | Mapping human microbiome drug metabolism by gut bacteria and their genes. Nature. 2019 Jun;570(7762):462-467. | |||||
| 4 | Metabolism, excretion, and pharmacokinetics of duloxetine in healthy human subjects | |||||
| 5 | DrugBank(Pharmacology-Metabolism):Duloxetine | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.

