| General Information of Drug (ID:
DR0854) |
| Drug Name |
Idelalisib
|
| Synonyms |
Idelalisib; YG57I8T5M0; Zydelig; CAL 101; CAL-101; CAL-101 (Idelalisib, GS-1101); CAL101; (S)-2-(1-((9H-Purin-6-yl)amino)propyl)-5-fluoro-3-phenylquinazolin-4(3H)-one; (S)-2-(1-(9H-purin-6-ylamino)propyl)-5-fluoro-3-phenylquinazolin-4(3H)-one; 1146702-54-6; 5-FLUORO-3-PHENYL-2-[(1S)-1-(9H-PURIN-6-YLAMINO)PROPYL]-4(3H)-QUINAZOLINONE; 5-Fluoro-3-phenyl-2-((S)-1-(9H-purin-6-ylamino)-propyl)-3H-quinazolin-4-one; 870281-82-6; CHEBI:82701; CHEMBL2216870; GS-1101; UNII-YG57I8T5M0
|
| Indication |
Chronic lymphocytic leukaemia
[ICD11: 2A82]
|
Approved
|
[1]
|
| Structure |
|
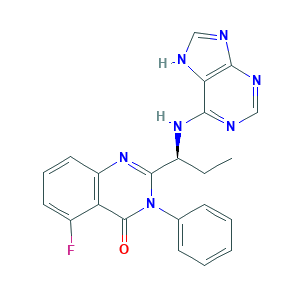 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
415.4 |
Topological Polar Surface Area |
99.2 |
| Heavy Atom Count |
31 |
Rotatable Bond Count |
5 |
| Hydrogen Bond Donor Count |
2 |
Hydrogen Bond Acceptor Count |
7 |
| Cross-matching ID |
- PubChem CID
- 11625818
- PubChem SID
-
16728852
; 78058152
; 124360767
; 124757878
; 125164680
; 135294860
; 136023431
; 136340110
; 136350004
; 136367521
; 136368015
; 137275917
; 141870606
; 144115773
; 152258425
; 160647261
; 160778577
; 162011391
; 162038002
; 162202617
; 163315619
; 163352092
; 163642790
; 163908064
; 164043527
; 164193919
; 170497648
; 170501514
; 172650954
; 172913174
; 174531110
; 177749172
; 178103352
; 184826685
; 198954688
; 198994003
; 215785737
; 223447422
; 223471406
; 223573729
; 223705059
; 224220340
; 225144930
; 226689936
; 248327652
; 249737291
; 249814532
; 249828711
; 250183703
; 250220450
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0J5VR
- Formula
- C22H18FN7O
- Canonical SMILES
- CCC(C1=NC2=C(C(=CC=C2)F)C(=O)N1C3=CC=CC=C3)NC4=NC=NC5=C4NC=N5
- InChI
- 1S/C22H18FN7O/c1-2-15(28-20-18-19(25-11-24-18)26-12-27-20)21-29-16-10-6-9-14(23)17(16)22(31)30(21)13-7-4-3-5-8-13/h3-12,15H,2H2,1H3,(H2,24,25,26,27,28)/t15-/m0/s1
- InChIKey
- IFSDAJWBUCMOAH-HNNXBMFYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


