| Synonyms |
Ketoralac; Ketorolac [INN:BAN]; Ketorolaco; Ketorolaco [Spanish]; Ketorolacum; Ketorolacum [Latin]; Macril; OZWKMVRBQXNZKK-UHFFFAOYSA-N; RS 37619; RS-37619; Toradol (TN); ketorolac; (+-)-5-Benzoyl-2,3-dihydro-1H-pyrrolizine-1-carboxylic acid; (+-)-Ketorolac; 1H-Pyrrolizine-1-carboxylic acid, 5-benzoyl-2,3-dihydro-; 5-(phenylcarbonyl)-2,3-dihydro-1H-pyrrolizine-1-carboxylic acid; 5-Benzoyl-2,3-dihydro-1H-pyrrolizine-1-carboxylic acid; 66635-83-4; 74103-06-3; CHEBI:76223
|
| Cross-matching ID |
- PubChem CID
- 3826
- PubChem SID
-
9274
; 5316934
; 7979681
; 8152424
; 12013083
; 14774471
; 29222945
; 46507019
; 48416148
; 49698948
; 50040043
; 50559242
; 53787558
; 56313647
; 57322012
; 85787389
; 87245106
; 90341692
; 92719330
; 96024794
; 99281833
; 103168363
; 103922837
; 104304637
; 118314823
; 124749910
; 124896716
; 125357028
; 126449597
; 126449602
; 126621065
; 126628266
; 126648434
; 126657726
; 126670368
; 126670856
; 128885449
; 131939830
; 134337908
; 135011243
; 135039632
; 136351611
; 137035346
; 142342166
; 160654547
; 160963811
; 162178870
; 162631206
; 163414850
; 164827307
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0D9JW
- Formula
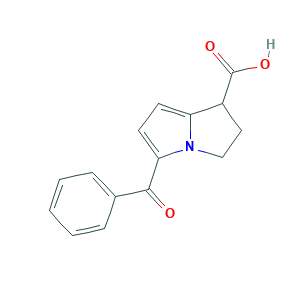
- C15H13NO3
- Canonical SMILES
- C1CN2C(=CC=C2C(=O)C3=CC=CC=C3)C1C(=O)O
- InChI
- 1S/C15H13NO3/c17-14(10-4-2-1-3-5-10)13-7-6-12-11(15(18)19)8-9-16(12)13/h1-7,11H,8-9H2,(H,18,19)
- InChIKey
- OZWKMVRBQXNZKK-UHFFFAOYSA-N
|