| Synonyms |
Meloxicam (Mobic); Meloxicamum; Meloxicamum [Latin]; Metacam; Mobicox; Movalis; Movatec; Parocin; Tenaron; UH-AC 62XX; UHAC-62XX; VG2QF83CGL; meloxicam; 4-Hydroxy-2-methyl-N-(5-methyl-2-thiazolyl)-2H-1,2-benzothiazine-3-carboxamide 1,1-dioxide; 4-Hydroxy-2-methyl-N-(5-methylthiazol-2-yl)-2H-benzo[e][1,2]thiazine-3-carboxamide 1,1-dioxide; 4-hydroxy-2-methyl-N-(5-methyl-1,3-thiazol-2-yl)-2H-1,2-benzothiazine-3-carboxamide 1,1-dioxide; 71125-38-7; C14H13N3O4S2; CCRIS 9139; CHEMBL599; MLS000028587; Mobec; Mobic; UNII-VG2QF83CGL
|
| Cross-matching ID |
- PubChem CID
- 54677470
- PubChem SID
-
10369
; 855815
; 6681236
; 7848032
; 7979894
; 11364527
; 11367089
; 11369651
; 11371577
; 11374373
; 11377813
; 11484638
; 11488757
; 11490465
; 11492602
; 11495447
; 11528628
; 12013466
; 14754262
; 26612637
; 26680359
; 26747003
; 26747004
; 26759123
; 29223163
; 39290017
; 39404337
; 42685351
; 46506624
; 47216906
; 47589118
; 47589119
; 47736629
; 48259389
; 48413967
; 49834840
; 50085983
; 50100965
; 53598948
; 53598949
; 53598950
; 53790511
; 56313654
; 56320470
; 57358017
; 81093329
; 85087488
; 85174237
; 87560523
; 89736106
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0G7FJ
- Formula
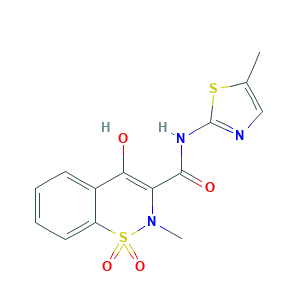
- C14H13N3O4S2
- Canonical SMILES
- CC1=CN=C(S1)NC(=O)C2=C(C3=CC=CC=C3S(=O)(=O)N2C)O
- InChI
- 1S/C14H13N3O4S2/c1-8-7-15-14(22-8)16-13(19)11-12(18)9-5-3-4-6-10(9)23(20,21)17(11)2/h3-7,18H,1-2H3,(H,15,16,19)
- InChIKey
- ZRVUJXDFFKFLMG-UHFFFAOYSA-N
|