| General Information of Drug (ID:
DR1315) |
| Drug Name |
Ponatinib hydrochloride
|
| Synonyms |
Ponatinib (AP24534); Ponatinib (HCl salt); Ponatinib HCl; Ponatinib Mono-hydrochloride; Ponatinib hydrochloride; Ponatinib hydrochloride [USAN]; 1114544-31-8; 96R6PU3D8J; AOB3899; AP 24534 hydrochloride; AP-24534 HCl; AP24534 HCL; AP24534 Hydrochloride; AS-16772; BCP10906; BWTNNZPNKQIADY-UHFFFAOYSA-N; C29H28ClF3N6O; CHEMBL2105708; D09951; DTXSID50149655; MFCD17215406; Ponatinib hydrochloride (JAN/USAN); SCHEMBL15798559; UNII-96R6PU3D8J; Y1466; Iclusig (TN); 3-(Imidazo[1,2-B]pyridazin-3-Ylethynyl)-4-Methyl-N-{4-[(4-Methylpiperazin-1-Yl)methyl]-3-(Trifluoromethyl)phenyl}benzamide; 3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N-(4-((4-methylpiperazin-1-yl)methyl)-3-(trifluoromethyl)phenyl)benzamide; 4340891KFS; 943319-70-8; AP 24534; AP-24534; AP24534; AP24534(Ponatinib); C29H27F3N6O; CHEBI:78543; CHEMBL1171837; UNII-4340891KFS; PONATINIB
|
| Indication |
Acute lymphoblastic leukemia
[ICD11: 2B33]
|
Approved
|
[1]
|
| Structure |
|
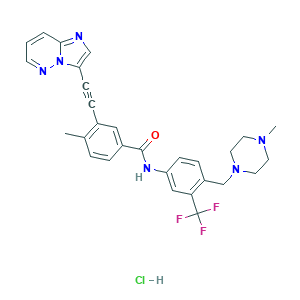 |
|
3D MOL is unavailable
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
569 |
Topological Polar Surface Area |
65.8 |
| Heavy Atom Count |
40 |
Rotatable Bond Count |
6 |
| Hydrogen Bond Donor Count |
2 |
Hydrogen Bond Acceptor Count |
8 |
| Cross-matching ID |
- PubChem CID
- 46908927
- CAS Number
-
- TTD Drug ID
- D0H0EQ
- Formula
- C29H28ClF3N6O
- Canonical SMILES
- CC1=C(C=C(C=C1)C(=O)NC2=CC(=C(C=C2)CN3CCN(CC3)C)C(F)(F)F)C#CC4=CN=C5N4N=CC=C5.Cl
- InChI
- 1S/C29H27F3N6O.ClH/c1-20-5-6-22(16-21(20)8-10-25-18-33-27-4-3-11-34-38(25)27)28(39)35-24-9-7-23(26(17-24)29(30,31)32)19-37-14-12-36(2)13-15-37;/h3-7,9,11,16-18H,12-15,19H2,1-2H3,(H,35,39);1H
- InChIKey
- BWTNNZPNKQIADY-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


