| Cross-matching ID |
- PubChem CID
- 121892
- PubChem SID
-
854071
; 10239851
; 12015005
; 14800601
; 24263039
; 29302387
; 49898865
; 50233601
; 53788352
; 57339830
; 58107328
; 85174557
; 85202062
; 91615883
; 92715048
; 103220716
; 103875964
; 104032719
; 104414526
; 118046799
; 118855330
; 124490309
; 125341609
; 126623656
; 126655065
; 126665918
; 128896436
; 131299831
; 134338628
; 135077118
; 135650896
; 136345679
; 136920410
; 136946473
; 137156938
; 141970640
; 152034730
; 152159608
; 160645840
; 162011781
; 162205078
; 163123042
; 163620909
; 163686230
; 164042455
; 164194995
; 164233343
; 164786707
; 174006313
; 174527464
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0X7GL
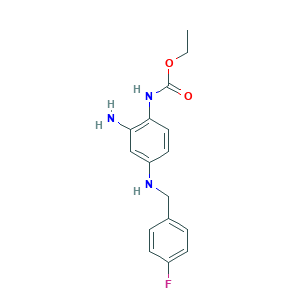
- Formula
- C16H18FN3O2
- Canonical SMILES
- CCOC(=O)NC1=C(C=C(C=C1)NCC2=CC=C(C=C2)F)N
- InChI
- 1S/C16H18FN3O2/c1-2-22-16(21)20-15-8-7-13(9-14(15)18)19-10-11-3-5-12(17)6-4-11/h3-9,19H,2,10,18H2,1H3,(H,20,21)
- InChIKey
- PCOBBVZJEWWZFR-UHFFFAOYSA-N
|