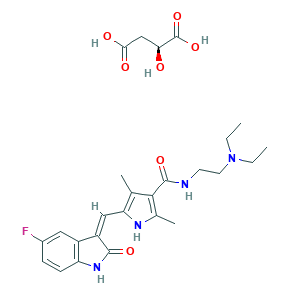
| Synonyms |
Sunitinib malate [USAN]; Sunitinib; Sunitinib Base; UNII-V99T50803M; V99T50803M; sunitinibum; 342641-94-5; 557795-19-4; CHEBI:38940; CHEMBL535; N-[2-(diethylamino)ethyl]-5-[(Z)-(5-fluoro-2-oxo-1,2-dihydro-3H-indol-3-ylidene)methyl]-2,4-dimethyl-1H-pyrrole-3-carboxamide; N-[2-(diethylamino)ethyl]-5-{[(3Z)-5-fluoro-2-oxo-2,3-dihydro-1H-indol-3-ylidene]methyl}-2,4-dimethyl-1H-pyrrole-3-carboxamide; NSC750690; SU11248; Su-011248; UNII-LVX8N1UT73; (Z)-N-(2-(diethylamino)ethyl)-5-((5-fluoro-2-oxoindolin-3-ylidene)methyl)-2,4-dimethyl-1H-pyrrole-3-carboxamide (S)-2-hydroxysuccinate; 341031-54-7; LVX8N1UT73; MFCD08282795; N-(2-(Diethylamino)ethyl)-5-((Z)-(5-fluoro-1,2-dihydro-2-oxo-3H-indol-3-ylidene)methyl)-2,4-dimethyl-1H-pyrrole-3-carboxamide (2S)-hydroxybutanedioate; PHA-290940AD; SU 011248; SU010398; SU011248; SU011248 L-malate salt; SUNITINIB MALEATE
|
| Cross-matching ID |
- PubChem CID
- 6456015
- CAS Number
-
- TTD Drug ID
- D0R0MW
- Formula
- C26H33FN4O7
- Canonical SMILES
- CCN(CC)CCNC(=O)C1=C(NC(=C1C)C=C2C3=C(C=CC(=C3)F)NC2=O)C.C(C(C(=O)O)O)C(=O)O
- InChI
- 1S/C22H27FN4O2.C4H6O5/c1-5-27(6-2)10-9-24-22(29)20-13(3)19(25-14(20)4)12-17-16-11-15(23)7-8-18(16)26-21(17)28;5-2(4(8)9)1-3(6)7/h7-8,11-12,25H,5-6,9-10H2,1-4H3,(H,24,29)(H,26,28);2,5H,1H2,(H,6,7)(H,8,9)/b17-12-;/t;2-/m.0/s1
- InChIKey
- LBWFXVZLPYTWQI-IPOVEDGCSA-N
|