| Cross-matching ID |
- PubChem CID
- 35370
- PubChem SID
-
9419
; 595886
; 596433
; 596437
; 596594
; 596595
; 597291
; 597292
; 597293
; 597457
; 597458
; 597459
; 597460
; 597461
; 597462
; 597463
; 597555
; 597641
; 597664
; 597665
; 600904
; 601536
; 602048
; 622272
; 624122
; 643735
; 644084
; 855980
; 7846195
; 7847479
; 7980906
; 8149829
; 8173993
; 11335699
; 11360938
; 11364242
; 11366804
; 11369366
; 11372752
; 11373842
; 11377528
; 11461910
; 11484570
; 11488743
; 11491322
; 11492147
; 11495162
; 12013681
; 12146009
; 14799161
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D01XYJ
- Formula
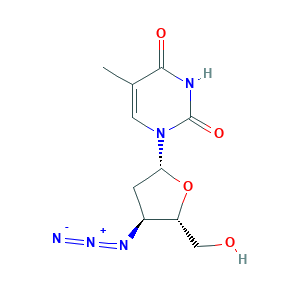
- C10H13N5O4
- Canonical SMILES
- CC1=CN(C(=O)NC1=O)C2CC(C(O2)CO)N=[N+]=[N-]
- InChI
- 1S/C10H13N5O4/c1-5-3-15(10(18)12-9(5)17)8-2-6(13-14-11)7(4-16)19-8/h3,6-8,16H,2,4H2,1H3,(H,12,17,18)/t6-,7+,8+/m0/s1
- InChIKey
- HBOMLICNUCNMMY-XLPZGREQSA-N
|