| Synonyms |
Zolpidem [INN:BAN]; Zolpidemum; Zolpidemum [Latin]; Zolpidem; 7K383OQI23; 82626-48-0; CHEBI:10125; CHEMBL911; DEA No. 2783; Lorex; N,N,6-Trimethyl-2-(4-methylphenyl)imidazo(1,2-a)pyridine-3-acetamide; N,N,6-Trimethyl-2-(4-methylphenyl)imidazo[1,2-a]pyridine-3-acetamide; N,N,6-Trimethyl-2-p-tolylimidazo[1,2-a]pyridine-3-acetamide; N,N-Dimethyl-2-[6-methyl-2-(4-methylphenyl)imidazo[1,2-a]pyridin-3-yl]acetamide; NCGC00095179-01; SL-800750; UNII-7K383OQI23; ZAFYATHCZYHLPB-UHFFFAOYSA-N; 2,3-bis(oxidanyl)butanedioic acid; 99294-93-6; A845992; ACT03353; AK126641; AKOS016013007; AX8157421; BCP28445; CCG-100938; CHEMBL1723343; CPD000469145; HMS2051H09; HMS2232G04; HMS3374C07; HMS3393H09; Intermezzo; MLS001401453; N,N-Dimethyl-2-(6-methyl-2-(p-tolyl)imidazo[1,2-a]pyridin-3-yl)acetamide 2,3-dihydroxysuccinate; N,N-dimethyl-2-[6-methyl-2-(4-methylphenyl)imidazo[1,2-a]pyridin-3-yl]ethanamide; NC00188; NYVVVBWEVRSKIU-UHFFFAOYSA-N; SAM001246549; SCHEMBL40721; SMR000469145
|
| Cross-matching ID |
- PubChem CID
- 18004026
- CAS Number
-
- TTD Drug ID
- D0T1WN
- Formula
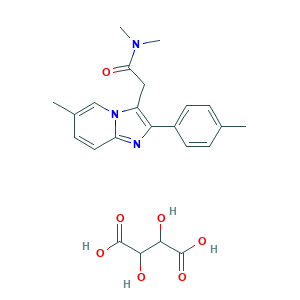
- C23H27N3O7
- Canonical SMILES
- CC1=CC=C(C=C1)C2=C(N3C=C(C=CC3=N2)C)CC(=O)N(C)C.C(C(C(=O)O)O)(C(=O)O)O
- InChI
- 1S/C19H21N3O.C4H6O6/c1-13-5-8-15(9-6-13)19-16(11-18(23)21(3)4)22-12-14(2)7-10-17(22)20-19;5-1(3(7)8)2(6)4(9)10/h5-10,12H,11H2,1-4H3;1-2,5-6H,(H,7,8)(H,9,10)
- InChIKey
- NYVVVBWEVRSKIU-UHFFFAOYSA-N
|