| General Information of Drug (ID:
DR0264) |
| Drug Name |
Candesartan cilexetil
|
| Prodrug Info |
Candesartan cilexetil is the prodrug of Candesartan
|
| Synonyms |
Candesartan; Candesartan (Cilexetil); Candesartan cilexetil; Candesartan cilexetil [USAN]; Kenzen; MFCD00871371; Parapres; TCV 116; TCV-116; TCY 116; Candesartancilexetil; Blopress; C24H20N6O3; CHEBI:3347; CHEMBL1016; CV 11974; CV-11974; NCGC00167474-01; Ratacand; S8Q36MD2XX; UNII-S8Q36MD2XX; 1-((2'-(1H-Tetrazol-5-yl)-[1,1'-biphenyl]-4-yl)methyl)-2-ethoxy-1H-benzo[d]imidazole-7-carboxylic acid; 139481-59-7; 2-Ethoxy-1-(p-(o-1H-tetrazol-5-ylphenyl)benzyl)-7-benzimidazolecarboxylic acid; 2-ethoxy-3-[[4-[2-(1h-tetrazol-5-yl)phenyl]phenyl]methyl]-3h-benzoimidazole-4-carboxylic acid; 2-ethoxy-3-[[4-[2-(2H-tetrazol-5-yl)phenyl]phenyl]methyl]benzimidazole-4-carboxylic acid; AK-57139; 145040-37-5; 1H-Benzimidazolium, 7-carboxy-1-(2-((cyclohexylcarbonyl)oxy)ethyl)-2-ethoxy-1-(2'-(1H-tetrazol-5-yl)(1,1'-biphenyl)-4-yl)-, hydroxide, inner salt, (+-)-; AK-75900; Amias; Atacand; C33H34N6O6; CANDESARTAN CILEXTIL; CHEBI:3348; CHEMBL1014
|
| Indication |
Essential hypertension
[ICD11: BA00]
|
Approved
|
[1]
|
| Structure |
|
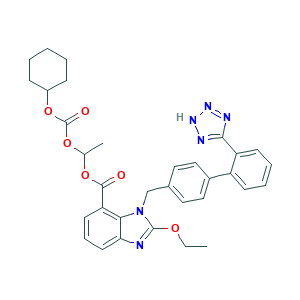 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
610.7 |
Topological Polar Surface Area |
143 |
| Heavy Atom Count |
45 |
Rotatable Bond Count |
13 |
| Hydrogen Bond Donor Count |
1 |
Hydrogen Bond Acceptor Count |
10 |
| Cross-matching ID |
- PubChem CID
- 2540
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0D5SQ
- Formula
- C33H34N6O6
- Canonical SMILES
- CCOC1=NC2=CC=CC(=C2N1CC3=CC=C(C=C3)C4=CC=CC=C4C5=NNN=N5)C(=O)OC(C)OC(=O)OC6CCCCC6
- InChI
- 1S/C33H34N6O6/c1-3-42-32-34-28-15-9-14-27(31(40)43-21(2)44-33(41)45-24-10-5-4-6-11-24)29(28)39(32)20-22-16-18-23(19-17-22)25-12-7-8-13-26(25)30-35-37-38-36-30/h7-9,12-19,21,24H,3-6,10-11,20H2,1-2H3,(H,35,36,37,38)
- InChIKey
- GHOSNRCGJFBJIB-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


