| Synonyms |
Dapagliflozin S-propylene glycol monohydrate; Dapagliflozin mixture with propylene glycol, hydrate; Dapagliflozin propanediol; Dapagliflozin propanediol [USAN]; Dapagliflozin propanediol hydrate; Dapagliflozin propylene glycol hydrate; Dapagliflozin propylene glycolate hydrate; UNII-887K2391VH; Dapagliflozin (S)-propylene glycol hydrate; Dapagliflozin propanediol monohydrate; Dapagliflozin; Forxiga; Forxiga (TN); UNII-1ULL0QJ8UC; (1S)-1,5-Anhydro-1-C-[4-chloro-3-[(4-ethoxyphenyl)methyl]phenyl]-D-glucitol; (2S,3R,4R,5S,6R)-2-(4-Chloro-3-(4-ethoxybenzyl)phenyl)-6- (hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol; (2S,3R,4R,5S,6R)-2-(4-chloro-3-(4-ethoxybenzyl)phenyl)-6-(hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol; 1ULL0QJ8UC; 461432-26-8; BMS 512148; BMS-512148; BMS512148; CHEBI:85078; CHEMBL3125458; CHEMBL429910; 887K2391VH; 960404-48-2; AK322430; BMS-512148-05; CHEBI:85079; Dapagliflozin ((2S)-1,2-propanediol, hydrate)
|
| Cross-matching ID |
- PubChem CID
- 9887712
- PubChem SID
-
14855234
; 15451267
; 24173975
; 45634651
; 49688954
; 57304407
; 57373691
; 76500746
; 96025580
; 103578280
; 104253204
; 109692963
; 123051086
; 124757330
; 124772065
; 125164134
; 126667088
; 126731318
; 134338792
; 134358357
; 136348250
; 136367545
; 136367974
; 137261282
; 140046947
; 143499356
; 144115882
; 152234916
; 152258220
; 160647056
; 162011553
; 162037657
; 162172238
; 164838825
; 165245557
; 170497930
; 174529446
; 175427068
; 178101315
; 185978787
; 194946819
; 198934677
; 198991956
; 210024059
; 211535346
; 221678783
; 223471387
; 223554897
; 223670709
; 223701082
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D01TNW
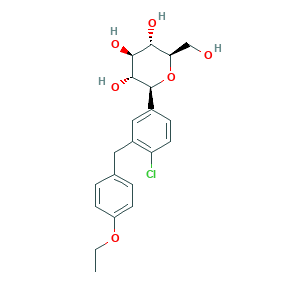
- Formula
- C21H25ClO6
- Canonical SMILES
- CCOC1=CC=C(C=C1)CC2=C(C=CC(=C2)C3C(C(C(C(O3)CO)O)O)O)Cl
- InChI
- 1S/C21H25ClO6/c1-2-27-15-6-3-12(4-7-15)9-14-10-13(5-8-16(14)22)21-20(26)19(25)18(24)17(11-23)28-21/h3-8,10,17-21,23-26H,2,9,11H2,1H3/t17-,18-,19+,20-,21+/m1/s1
- InChIKey
- JVHXJTBJCFBINQ-ADAARDCZSA-N
|