| Synonyms |
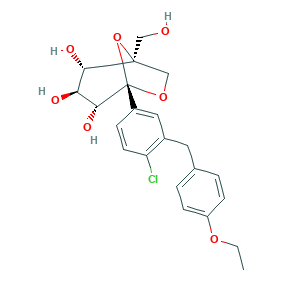
Ertugliflozin; Ertugliflozin (PF 04971729); Ertugliflozin [USAN:INN]; MK-8835; PF 04971729; PF-04971729; PF04971729; Steglatro; (1S,2S,3S,4R,5S)-5-(4-chloro-3-(4-ethoxybenzyl)phenyl)-1-(hydroxymethyl)-6,8-dioxabicyclo[3.2.1]octane-2,3,4-triol; (1S,2S,3S,4R,5S)-5-[4-chloro-3-[(4-ethoxyphenyl)methyl]phenyl]-1-(hydroxymethyl)-6,8-dioxabicyclo[3.2.1]octane-2,3,4-triol; 1,6-Anhydro-1-C-[4-chloro-3-[(4-ethoxyphenyl)methyl]phenyl]-5-C-(hydroxymethyl)-beta-L-idopyranose; 1210344-57-2; 6C282481IP; CHEMBL1770248; UNII-6C282481IP
|
| Cross-matching ID |
- PubChem CID
- 44814423
- PubChem SID
-
89442948
; 120130619
; 131281547
; 131345821
; 140604034
; 162011560
; 162108670
; 162205143
; 163312343
; 174526435
; 185978788
; 198984080
; 210024075
; 223404370
; 223471394
; 224604107
; 226541727
; 243511761
; 252109952
; 252166588
; 252448906
- CAS Number
-
- TTD Drug ID
- D0Q3VE
- Formula
- C22H25ClO7
- Canonical SMILES
- CCOC1=CC=C(C=C1)CC2=C(C=CC(=C2)C34C(C(C(C(O3)(CO4)CO)O)O)O)Cl
- InChI
- 1S/C22H25ClO7/c1-2-28-16-6-3-13(4-7-16)9-14-10-15(5-8-17(14)23)22-20(27)18(25)19(26)21(11-24,30-22)12-29-22/h3-8,10,18-20,24-27H,2,9,11-12H2,1H3/t18-,19-,20+,21-,22-/m0/s1
- InChIKey
- MCIACXAZCBVDEE-CUUWFGFTSA-N
|